Posted inMaterial Science
Ferromagnetism
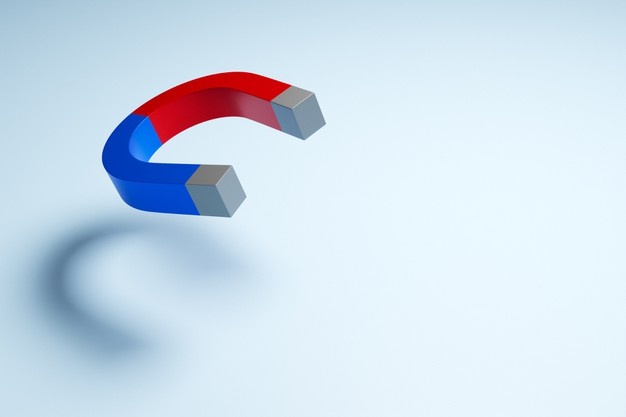
Certain metallic materials possess a permanent magnetic moment in the absence of an external field, and manifest very large and permanent magnetizations. These are the characteristics of ferromagnetism, and they are displayed by the transition metals iron, cobalt, nickel, and some of the rare earth metals. Permanent magnetic moments in ferromagnetic materials result from atomic magnetic moments due to electron spinuncancelled electron spins as a consequence of the electron structure. There is also an orbital magnetic moments contribution that is small in comparison to the spin moment. Furthermore, in a ferromagnetic material, coupling interactions cause net spin magnetic moments of adjacent atoms to align with one another, even in the absence of an external field. The maximum possible magnetization or saturation magnetization Ms of a ferromagnetic material represents the magnetization that results when all the magnetic diploes in a solid piece are mutually aligned with the external field; there is also a corresponding saturation flux density Bs. Iron, nickel, and cobalt are examples of ferromagnetic materials.