Posted inMaterial Science
Corrosion of Ceramics
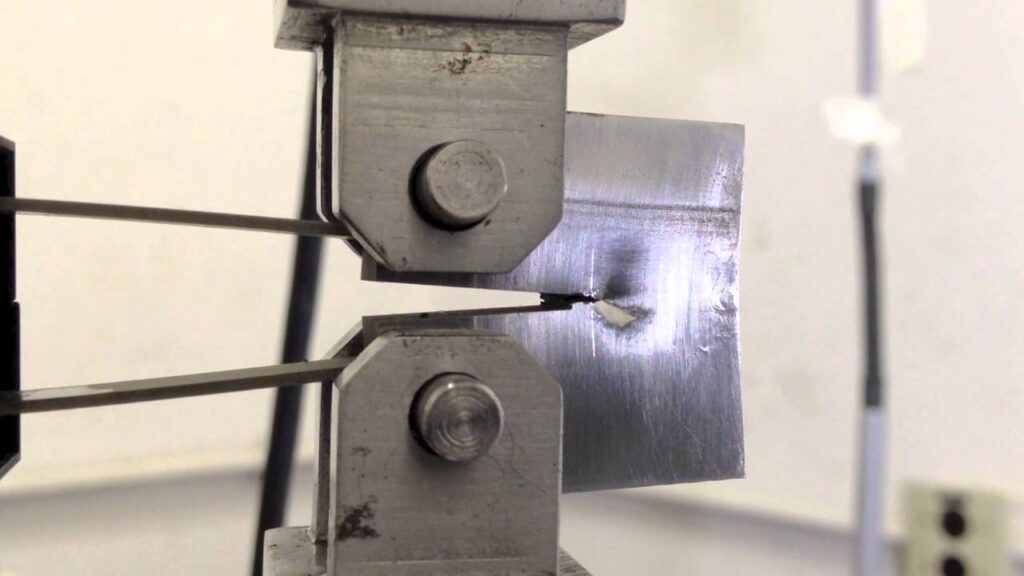
It is often said that one of the biggest advantages which ceramics have over other materials is their corrosion resistance, that is, their chemical inertness in corrosive environments. Is this always true? Corrosion is generally understood as property degradation due to environmental attack. As it will be shown in this section, there are a number of environments in which ceramics can degrade at a rapid rate. There exists a tremendous need for reliable and corrosion resistant structural ceramic or partly ceramic materials which can be used in aggressive environments such as: - high energy battery systems (such as sodium-sulphur): beta-alumina is being investigated - gas turbines: silicon nitride and/or carbide are being investigated - heat exchangers: SiC, composites are being investigated Ceramics are indeed much more environmentally…