Geometrical Shape
Crystalline Solids: Crystalline solids have a well-defined geometrical shape due to the regular arrangement of unit cells.
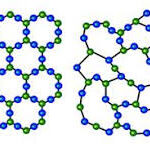
Noncrystalline Solids: Non-crystalline solids do not have well–defined geometrical shape.
Range Order
Crystalline Solids: Crystalline solids have a long range order.
Noncrystalline Solids: Non-crystalline solids have a short range order.
Melting Point
Crystalline Solids: Crystalline solids have a definite melting point.
Noncrystalline Solids: Non-crystalline solids melt over a range.
Heat of Fusion
Crystalline Solids: Crystalline solids have a high fixed value for the heat of fusion.
Noncrystalline Solids: Non-crystalline solids do not have a fixed value for the heat of fusion.
Properties of Solids
Crystalline Solids: Crystalline solids are true solids. They show all the properties of solids.
Noncrystalline Solids: Non-crystalline solids do not show all the properties of solids. Therefore, they are called “pseudo solids”.
Energy
Crystalline Solids: Energy in crystalline solids is lower than that of non-crystalline solids.
Noncrystalline Solids: Nature favours crystalline solids due to the low energy arrangement.