Crystalline Solids and Non-crystalline Solids are the two main categories of solids that show some difference between them in terms of the arrangement of the constituent particles and other properties. The key difference between crystalline solids and non-crystalline solids is that Crystalline Solids have an evenly distributed three-dimensional arrangement of atoms, ions, or molecules while Non crystalline Solids do not have a consistent arrangement of atoms.
What is Crystalline Solid?
In crystalline solids, constituent particles (atoms, molecules or ions) are arranged in a three-dimensional periodic manner. They are bounded by planes or faces. The smallest repeating unit in crystalline solids is called “unit cell”. All the unit cells in a particular solid are identical and repeating. For example; unit cells can be considered as bricks in a wall.
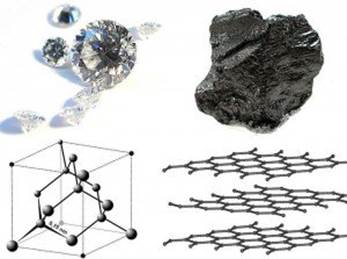
Diamond and Graphite: Examples of Crystalline Solids
Crystalline solids can also be categorized as follows.
| Type | Constituents | Inter molecular Forces | Properties |
| Ionic Solids (Table salt – NaCl) | Positive and negative ions | Electrostatic attractions | Very high melting points, Poor conductors, Brittle |
| Molecular Solids (Sucrose) | Atoms and molecules | London dispersion forces and Dipole-Dipole attractions, Hydrogen bonds | Low melting point, Flexible, Poor conductors |
| Covalent Network (graphite, diamond) | Atoms | Covalent bonds, Weak London forces | Very high melting and boiling points, Poor conductors |
| Metallic Solids | Metal atoms | Metallic bonds | High melting point, Soft-malleable, Very hard, Good conductors |
What is Noncrystalline Solid?
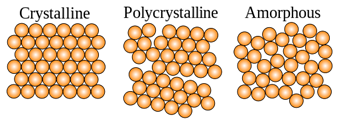 Non- crystalline solids are also known as “amorphous solids”. Unlike crystalline solids, they do not have a definite geometrical shape. In solids, atoms are closely held together more than liquids and gases. However, in non-crystalline solids, particles have a little freedom to move since they are not arranged rigidly as in crystalline solids. These solids are formed, after sudden cooling of a liquid. The most common examples are plastic and glass.
Non- crystalline solids are also known as “amorphous solids”. Unlike crystalline solids, they do not have a definite geometrical shape. In solids, atoms are closely held together more than liquids and gases. However, in non-crystalline solids, particles have a little freedom to move since they are not arranged rigidly as in crystalline solids. These solids are formed, after sudden cooling of a liquid. The most common examples are plastic and glass.
What is the difference between Crystalline and Non crystalline Solids?
Arrangement of Particles in Crystalline and Non crystalline Solids
Crystalline Solids: Crystalline Solids have an evenly distributed three-dimensional arrangement of atoms, ions, or molecules.
Non crystalline solids: Non-crystalline solids do not have a consistent arrangement of particles.