Thermal Energy
Energy can come in many forms, and it can change from one form to another but can never be lost. This is the First Law of Thermodynamics. A byproduct of nearly all energy conversion is heat, which is also known as thermal energy. When there is a temperature difference between two objects or two areas within the same object, heat transfer occurs. Heat energy transfers from the warmer areas to the cooler areas until thermal equilibrium is reached. This is the Second Law of Thermodynamics. When the temperature of an object is the same as the surrounding environment, it is said to be at ambient temperature.
Heat Transfer Mechanisms
Thermal energy transfer occurs through three mechanisms: conduction, convection, and/or radiation. Conduction occurs primarily in solids and to a lesser degree in fluids as warmer, more energetic molecules transfer their energy to cooler adjacent molecules. Convection occurs in liquids and gases, and involves the mass movement of molecules such as when stirring or mixing is involved.
The third way that heat is transferred is through electromagnetic radiation of energy. Radiation needs no medium to flow through and, therefore, can occur even in a vacuum. Electromagnetic radiation is produced when electrons lose energy and fall to a lower energy state. Both the wavelength and intensity of the radiation is directly related to the temperature of the surface molecules or atoms.
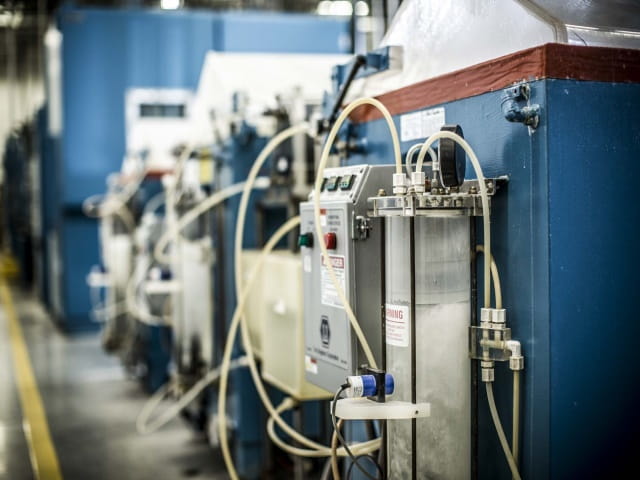
Wavelength of Thermal Energy
The wavelength of thermal radiation extends from 0.1 microns to several hundred microns. As highlighted in the image, this means that not all of the heat radiated from an object will be visible to the human eye… but the heat is detectable. Consider the gradual heating of a piece of steel. With the application of a heat source, heat radiating from the part is felt long before a change in color is noticed. If the heat intensity is great enough and applied for long enough, the part will gradually change to a red color. The heat that is felt prior to the part changing color is the radiation that lies in the infrared frequency spectrum of electromagnetic radiation. Infrared (IR) radiation has a wavelength that is longer than visible light or, in other words, greater than 700 nanometers. As the wavelength of the radiation shortens, it reaches the point where it is short enough to enter the visible spectrum and can be detected with the human eye.
An infrared camera has the ability to detect and display infrared energy. Below is an infrared image of an ice cube melting. Note the temperature scale on side, which shows warm areas in red and cool areas in purple. It can be seen that the ice cube is colder than the surrounding air and it is absorbing heat at its surface. The basis for infrared imaging technology is that any object whose temperature is above 0°K radiates infrared energy. Even very cold objects radiate some infrared energy. Even though the object might be absorbing thermal energy to warm itself, it will still emit some infrared energy that is detectable by sensors. The amount of radiated energy is a function of the object’s temperature and its relative efficiency of thermal radiation, known as emissivity.

(Photo courtesy of NASA/JPL-Caltech/IPAC)
Emissivity
A very important consideration in radiation heat transfer is the emissivity of the object being evaluated. Emissivity is a measure of a surface’s efficiency in transferring infrared energy. It is the ratio of thermal energy emitted by a surface to the energy emitted by a perfect blackbody at the same temperature. A perfect blackbody only exists in theory and is an object that absorbs and reemits all of its energy. Human skin is nearly a perfect blackbody as it has an emissivity of 0.98, regardless of actual skin color.
If an object has low emissivity, IR instruments will indicate a lower temperature than the true surface temperature. For this reason, most systems and instruments provide the ability for the operator to adjust the emissivity of the object being measured. Sometimes, spray paints, powders, tape or “emissivity dots” are used to improve the emissivity of an object.
Equipment – Detectors
Thermal energy detection and measurement equipment comes in a large variety of forms and levels of sophistication. One way to categorize the equipment and materials is to separate thermal detectors from quantum (photon) detectors. The basic distinction between the two is that thermal detectors depend on a two-step process. The absorption of thermal energy in these detectors raises the temperature of the device, which in turn changes some temperature-dependent parameter, such as electrical conductivity. Quantum devices detect photons from infrared radiation. Quantum detectors are much more sensitive but require cooling to operate properly.
Thermal Detectors
Thermal detectors include heat sensitive coatings, thermoelectric devices and pyroelectric devices. Heat sensitive coatings range from simple wax-based substances that are blended to melt at certain temperatures to specially formulated paint and greases that change color as temperature changes. Heat sensitive coatings are relatively inexpensive but do not provide good quantitative data.

Thermoelectric devices include thermocouples, thermopiles (shown right), thermistors and bolometers. These devices produce an electrical response based on a change in temperature of the sensor. They are often used for point or localized measurement in a contact or near contact mode. However, thermal sensors can be miniaturized. For example, microbolometers are the active elements in some high-tech portable imaging systems, such as those used by fire departments. Benefits of thermal detectors are that the element does not need to be cooled and they are comparatively low in price. Thermal detectors are used to measure the temperature in everything from home appliances to fire and intruder detection systems to industrial furnaces to rockets.
Quantum (Photon) Detectors
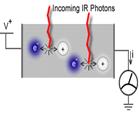
Unlike thermal detectors, quantum detectors do not rely on the conversion of incoming radiation to heat, but convert incoming photons directly into an electrical signal. When photons in a particular range of wavelengths are absorbed by the detector, they create free electron-hole pairs, which can be detected as electrical current. The signal output of a quantum detector is very small and is overshadowed by noise generated internally to the device at room temperatures. Since this noise within a semiconductor is partly proportional to temperature, quantum detectors are operated at cryogenic temperatures [i. e. down to 77 K (liquid nitrogen) or 4 K (liquid helium)] to minimize noise. This cooling requirement is a significant disadvantage in the use of quantum detectors. However, their superior electronic performance still makes them the detector of choice for the bulk of thermal imaging applications. Some systems can detect temperature differences as small as 0.07°C.
Quantum detectors can be further subdivided into photoconductive and photovoltaic devices. The function of photoconductive detectors are based on the photogeneration of charge carriers (electrons, holes or electron-hole pairs). These charge carriers increase the conductivity of the device material. Possible materials used for photoconductive detectors include indium antimonide (InSb), quantum well infrared photodetector (QWIP), mercury cadmium telluride (mercad, MCT), lead sulfide (PbS), and lead selenide (PbSe).
Photovoltaic devices require an internal potential barrier with a built-in electric field in order to separate photo-generated electron-hole pairs. Such potential barriers can be created by the use of p-n junctions or Schottky barriers. Examples of photovoltaic infrared detector types are indium antimonide (InSb), mercury cadmium telluride (MCT), platinum silicide (PtSi), and silicon Schottky barriers.
Detector Cooling
There are several different ways of cooling the detector to the required temperature. In the early days of thermal imaging, liquid nitrogen was poured into imagers to cool the detector. Although satisfactory, the logistical and safety implications led to the development of other cooling methods. High pressure gas can be used to cool a detector to the required temperatures. The gas is allowed to rapidly expand in the cooling systems and this expansion results in the significant reduction in the temperature of a gas. Mechanical cooling systems are the standard for portable imaging systems. These have the logistical advantage of freeing the detection system from the requirements of carrying high pressure gases or liquid nitrogen.