Size- and shape-controlled synthesis of self-organized, three-dimensional (3D) micro/nano architectures are still challenging and much attracted in the recent years owing to vast scientific and technological interest in multifaceted research areas [11, 27, 14]. The growth and development of uniform, self-aggregated 3D super-structures through plane-to-plane coalition of the basic building blocks have been paid significant attention owing to their unusual physical, chemical properties [29, 3]. The development of self-organized architectures consents to find neoteric and diversified materials, causing momentous discovery that has been developed for the construction of a new class of micro/nanostructured devices [14, 15]. Self-aggregation of hierarchical architectures has proved to be a virtuous and expedient tool to control the structure and properties for the fabrication of intricate networks [15]. The peculiar size and morphology of the self-assembled 3D networks determine the attributes of a material which are important for the manufacture of a novel category of micro/nano-structured devices for advanced engineering and sciences [29, 14, 13]. The supreme and hopeful approach to synthesize self-assembled 3D networks is to use a solution-based technique which is a simple route to regulate the physicochemical properties of materials [3]. Specifically, in the hydrothermal method, numerous versatile morphologies have been described by using surfactants or chelating agents [7, 16, 17]. The surface capping agent used in the hydrothermal synthesis method positively assures an effective way to regulate the nucleation, controlled growth, oriented aggregation mechanism, and self-organized 3D morphology [15, 4].
Recent research scenario
A wide variety of 3D super-structures can be synthesized by using a suitable amount of surfactant which reduces the particle–particle amalgamation by steric effects, thereby controlling the size and shape [15]. Up till now, a number of research works have been reported on controlled synthesis of 3D hierarchical architectures using hydrothermal technique, for example, bowknot-like Y2(WO4)3:Ln3+[9], almond-like (Na0.5La0.5)MoO4:RE3+ [14], monodisperse CaMoO4:Eu3+, M+ (M = Li, Na, K) microspheres [18], micron-sized flower-like NaCe(MoO4)2 architectures [27], tetragonal bipyramid-like (Na0.5Gd0.5)MoO4:Ln3+ [15], rugby-like NaEu(MoO4)2 microstructures [26], sheaf-like Gd2(MoO4)3[24], etc. Large-scale assert for phosphor materials as efficient sources of energy that can supply sustained proficiency is growing gradually. Current research in phosphors is focused on new materials having both luminescence and magnetic properties in a single entity based on the presence of trivalent lanthanide ions (Ln3+) with magnetic Fe3+ ions. These bifunctional materials have attracted worldwide intense research, because their up/down conversion luminescence and para/ferromagnetic properties are strongly required for therapeutics, contrast agents in magnetic resonance (MRI), fluorescent bioimaging in biophotonics, drug delivery and lumino-magnetic applications [6, 13, 19]. The phosphor particles can be magnetically directed, aligned, tracked, after applying the external magnetic fields. Subsequently, their luminescence can be visualized under optical excitation, which can be used widely for biological labeling, imaging of human and animal cells, tissues, and in vitro, in vivo applications [22, 25].
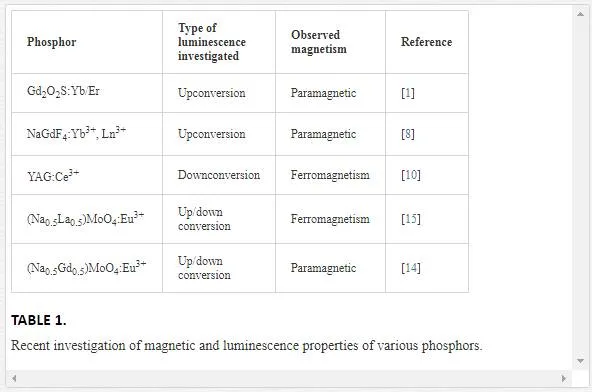
The luminescence and magnetic properties of lanthanide ions doped iron-based inorganic compounds that are synthesized by different research groups are detailed as follows. The novel Fe3O4@YPO4:Re (Re = Tb, Eu) magnetic-fluorescent hybrid spheres were prepared using solvothermal and co-precipitation techniques [25]. The obtained nanostructures are an excellent platform to integrate fluorescent materials and magnetite into one single entity to exhibit both magnetic and fluorescent properties [25]. Wang et al. [25] reported that human cervical carcinoma Hela cells were successfully labeled by using the Fe3O4@YPO4:Re hybrid spheres. Zhang et al. [29] synthesized orthorhombic cubic GdFeO3 particles by using a facile hydrothermal method. The emission color of cubic GdFeO3particles could be adjusted effectively by doping different lanthanide activators [29]. Also, cubic GdFeO3 particles exhibiting paramagnetic properties are reported to be used as an excellent luminescence and magnetic material [29]. To replace the existing halide-based upconverting phosphors for infrared-based biomedical imaging [1], nontoxic Gd2O2S:Yb/Er is synthesized using flux fusion method. The synthesized phosphor not only enables near-IR imaging features but also at the same time could be used as a contrast agent in MRI imaging [1]. Multifunctional luminescent-magnetic YAG:Ce nano-phosphors were synthesized by post-heat solidified combustion technique, post-heated hydrothermal-homogenous precipitation, and post-heated auto-clave techniques, respectively, using inexpensive aluminum and yttrium nitrates as the starting materials and urea as the homogenizing precipitant [10]. Since the yttrium and aluminium are non-magnetic elements, the origin of ferromagnetism of these nonmagnetic oxides could be attributed solely to the defects and/or oxygen vacancies [10].
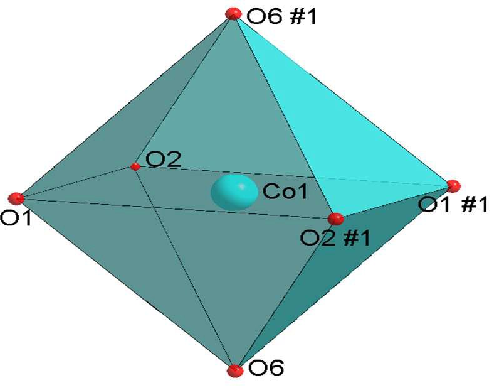
The novel YAG:Ce nanophosphor exhibits the ferromagnetic behavior which finds the way for a new class of magneto-optic and spintronic materials for a wide variety of applications. The multicolor upconversion emissions and paramagnetic nature were observed in β-NaGdF4 crystals, which were synthesized by one-step precipitation method at room temperature [8]. To control the crystal size and morphology of the β-NaGdF4 crystals, different types of surfactants, namely Na2EDTA, PVP, SDS, and Na2tar, were introduced during the synthesis procedure. Due to the presence of paramagnetic Gd3+ ions, β-NaGdF4 crystals exhibit paramagnetic behavior, which is a favorable bifunctional candidate for bioimaging applications [8]. Scheelite-type metal molybdates are considered as an ideal candidate, and are receiving accelerating interest due to their remarkable luminescence, magnetic, catalytic, sensing, and photocatalytic properties [13]. Scheelite-type crystalline structures, with the general chemical formula such as (Na0.5R0.5)MoO4:Ln3+ [15, 14]; BaMoO4:Ln3+ [23; Ca0.5R(MoO4)2:Ln3+ [20]; Fe0.5R0.5(MoO4)1.5:Ln3+ [13] (where R = La or Gd or Y and Ln = Eu, Tb, Dy, etc.) etc., where molybdenum atoms are coordinated tetrahedrally, have recently attracted great attention because of their promising applications in the opto-electronics [14, 15]. In the crystal structure of Fe0.5R0.5(MoO4)1.5:Ln3+, trivalent Fe3+, R3+/Ln3+ jointly occupy the dodecahedral positions and molybdenum (Mo6+) atoms populated at the center of the tetrahedral symmetry coordinated with four equivalent oxygen atoms. Scheelite-type metal molybdates with the general formula have been synthesized by various techniques, which include hydrothermal method [14, 15], solid-state reaction technique [12], hydrothermal-assisted solid-state reaction [28], and pulsed laser deposition technique [21]. The random distribution of R3+ (La or Gd or Y) in the scheelite tetragonal structures show an intense and broad absorption band in the near UV- region [15]. Among all these molybdates, (Fe0.5Gd0.5)MoO4:Eu3+ is an important opto-magnetic material and possess good luminescence property. Due to the notable emission spectral lines in Ln3+ doped phosphors extending from ultraviolet to infra-red regionand its magnetic properties may open up a new platform to design and formulate other inorganic functional materials with for potential magnetic and luminescence applications [13], there is a great deal of scientific and technological interest in developing novel opto-electronic applications.
In general, synthesis of any kind of phosphor materials using the conventional/sol–gel technique would require laborious conditions such as hefty manual grinding, annealing, or calcination of samples to high temperature, intermediate grinding process, and heat treatment for several times, etc. In order to avoid the above mentioned difficulties, hydrothermal technique is the alternative and most promising approach to synthesize the variety of phosphor materials with desired size, shape, and dimensions, crystallinity by changing the experimental parameters.Herein, we report the controlled synthesis of (Fe0.5Gd0.5)MoO4:Eu3+ microcrystals (self-aggregated 3D superstructures) using hydrothermal method by employing PVP as the surfactant. By varying the reaction time, we have reported the morphology selection and the condition to derive novel nanoparticle sheathed bipyramid-like morphology. The rest of the parameters like molar ratio between initial precursor / surfactant and temperature were kept as constant. The (Fe0.5Gd0.5)MoO4:Eu3+ microcrystals are found to be excellent matrix for photoluminescence property and the Eu3+ ion serves as a good red-emitting luminescent center. Further, room-temperature (RT) magnetic properties of Fe0.5Gd0.5(MoO4)1.5:Eu3+ were investigated in detail.
Synthesis, morphology, structure of the Fe0.5Gd0.5(MoO4)1.5:Eu3+ 3D hierarchical microstructures
SYNTHESIS PROCEDURE
All the chemicals were purchased from Sigma Aldrich with 99.99% purity. In the typical synthesis, a stoichiometric amount of FeCl3 6H2O was dissolved in 15 mL of double-distilled water through vigorous stirring. Then, the appropriate amount of Na2MoO4 was initially dissolved in 30 mL of double- distilled water under vigorous stirring. Then, GdCl3 and EuCl3 were prepared by dissolving the corresponding rare-earth sesquioxides (Gd2O3, Eu2O3) in diluted hydrochloric acid and stirred for 15 min. In order to remove the excess of HCl, the solution was heated to 60–80°C for 30 min. Then, the rare-earth chloride solution was carefully mixed into the Na2MoO4 solution. A white colloidal precipitate was obtained under vigorous stirring for 15 min. Subsequently, iron chloride solution was gradually introduced into the above white precipitate solution, forming a brown mixture solution. Then, 0.1 mM of polyvinyl pyrrolidone (PVP40) was dissolved in 20 mL of double-distilled water, and was added to the resultant colloidal solution. Then, the pH value of the final product was consequently, adjusted to a value of 7 -8 by adding the proper amount of sodium hydroxide solution. After additional stirring for 30 min, the acquired brown mixture solution was transferred into a 100 mL Teflon-lined stainless steel autoclave, and heated at 200°C for 6, 12, and 24 h. Finally, the autoclave was allowed to cool at room temperature, and the final product was centrifugally cleaned (5, 000 rpm for 30 min to remove organic containments and NaCl), separated from the solution, rinsed with distilled water and absolute ethanol several times, and dried at 60°C in the air for 5 h.
CHARACTERIZATION
XRD pattern of as-synthesized phosphor was analyzed using PANalytical’s X’Pert PRO materials research, X-ray diffractometer equipped with a Cu-Kα radiation (λ = 1.54060 A°) at a scanning rate of 0.02° s-1 performed in the range of 20–60 degrees. Morphology and energy-dispersive X-ray spectra (EDX) of the product were investigated by scanning electron microscope (FESEM (Quanta 3D FEG)). The spacing between the two adjacent lattice planes was measured using high-resolution transmission electron microscope (HRTEM JEOL 3010). Furthermore, the room-temperature (RT) photoluminescence studies were carried out using Horiba-Jobin Yvon Fluromax-4P bench-top Spectrofluorometer. Room-temperature magnetic properties of the samples were analyzed using (Lake Shore 7307 model) vibrating sample magnetometer (VSM).
MORPHOLOGICAL INVESTIGATIONS
To explore the 3D surface morphology, particle size distribution, and formation mechanism for the evolution of nanoparticle sheathed bipyramid-like structures, a series of time-dependent experiments were carried out using a PVP employed hydrothermal route.

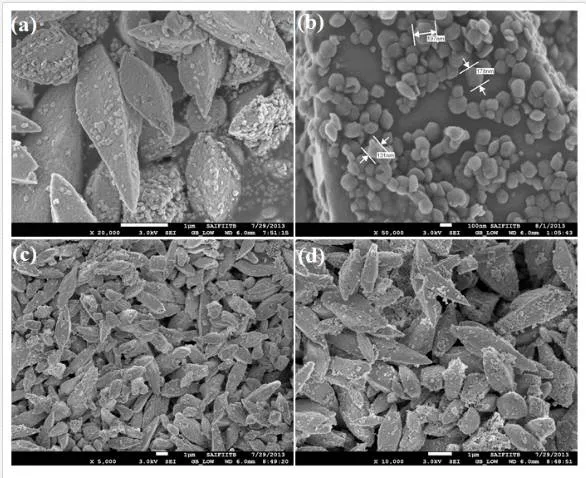
The FESEM and TEM analyses were used to characterize the morphology of the final products. By changing the experimental reaction time interval with fixed temperature (200°C), pH (~7), and molar concentration of the PVP (0.1 mM), a substantial difference was observed in the final product. Figure (1–3)shows the low, high magnifications FESEM image of the Fea0.5Gd0.5(MoO4)1.5:Eu3+ samples synthesized by hydrothermal route at different time intervals (1, 3, 6, 12, 24, and 48 h) with fixed temperature (200°C), pH (~7), and PVP molar concentration. During the initial period of 1 h time interval, numerous 2D nanoflakes with few tens of nanometer were observed, which acts as a primary building block for the subsequent growth of 3D microstructures (Figure 1 (a)). It was noticed from Figure 1 (b, c), when the hydrothermal time period was increased to 3 and 6 h, nanoflakes began to self-organize continuously through plane-to-plane coalition and try to form a bipyramidal morphology (average diameter of 1 μm). However, the morphology and size distribution was found to be less uniform. When the reaction time interval was kept at 12 h, bipyramid-like morphology with an average of 1.0 μm in length and an average of 1.2 μm in diameter was obtained and shown in Fig. 1 (d). When the reaction time period was raised to 24 h, highly ordered and self-assembled porous nanoparticle sheathed bipyramid-like morphology with the size approximately equal to ~1.2 μm diameter was observed (Figure 2 (a, b)). Further, increasing the reaction time to 48 h, the final product morphology consisted of irregular porous bipyramid-like structures in which nanoparticles are sheathed and shown in Figure 2 (c, d). This may be due to the prolonged time interval, resulting in thermal collision between the particles (growth stage; the self-assembly process predominates between these crystals because of its high surface energies) [14].
This involves growth from 0D primary particles to 2D nanosheets, followed by the formation of 3D hierarchical networks through an assembly–disassembly process [14]. The TEM image of the sample synthesized at 24 h time interval with 0.1 mM of PVP molar concentration is shown in Figure 3a and the corresponding inset shows the FFT pattern. The formation mechanism for the evolution of a nanoparticle-sheathed bipyramid-like Fe0.5Gd0.5(MoO4)1.5:Eu3+ structure can be described as follows. Primarily, smaller crystalline nuclei formed in a supersaturated solution starts the crystallization process for subsequent growth of self-organization. During the self-organization process, smaller nanocrystals were grown-up constantly to form bigger particles via an Ostwald ripening phenomenon at higher reaction time intervals, due to interactions between the amorphous nanoparticles, resulting in 2D nanoflakes [14]. Initially, PVP acts as a surface capping agent to form a stable complex with Gd3+/Fe3+and then kinetically controls the reaction rate, germination of nuclei, the growth rate, and the oriented aggregation mechanism. When the reaction temperature gradually increases to higher temperature, PVP would decompose gradually and has no obvious effect on the possible growth of nuclei. When the time period was increased, these 2D nanoflakes joined each other along a mutual crystallographic orientation through plane-to-plane conjunction of neighboring particles [14]. In general, such amalgamation process between the atoms/ions in molecules/crystals can induce dipole moments, which can be associated with certain specific crystallographic orientations [13]. The interaction among dipole moments in nearby nanoparticles is highly probable and acts as a driving factor for the oriented attachment procedure by allocation of a common crystallographic facet. The growth rate in Fe0Gd0.5(MoO4)1.5:Eu3+ crystals is oriented preferentially along the [001] direction. Donnay-Harker et al. [5] reported that, for tetragonal structures, the surface free energy of the [001] face is larger than that of the [101] face [5, 2]. As a result, the faster growth rate in the [100], [010], and [001] faces in comparison with that in the [101] direction accelerates the formation of self-assembled structures.

The as-grown 2D nanoflakes are continuously self-assembled via a layer-by-layer stacking process to form 3D bipyramid-like structures. Then, the completely developed bipyramid particles are readily disassembled into smaller nanoparticles, which are finally sheathed on individual bipyramid-like particles. After aging for a longer time period, the disassembly of well-organized structures could increase the entropy of the system and thus could decrease the free energy of the whole system [16], resulting in the nanoparticles arbitrarily sheathed on individual bipyramid-like particles. The assembly–disassembly process in Fe0Gd0.5(MoO4)1.5:Eu3+ was described in our previous work [13] by using EDTA as a surface capping agent.

The chemical phase purity and crystalline nature of the product were investigated using X-ray diffraction patterns. Figure 5 shows the XRD pattern of Fe0.5Gd0.5(MoO4)1.5:Eu3+ samples synthesized by a facile hydrothermal route at different time intervals (6, 12, 24, and 48 h). All the peaks are perfectly matched with the standard JCPDS card no. 25-0828 of (Na0.5Gd0.5)MoO4. It could be clearly observed that the XRD patterns of as-synthesized samples are highly crystalline, and the strongest intensity peak is observed at 2θ = 28.67 degrees analogous to (112) plane. The XRD pattern reveals that they belong to a tetragonal phase corresponding to the scheelite structure with the space group I41/a. In this crystal structure Fe3+, Gd3+/Eu3+ ions jointly occupy the dodecahedral sites of the tetrahedra. Molybdenum atoms (Mo6+) populated in the center of tetrahedral symmetry is surrounded by four equivalent oxygen (O2–) atoms [13] and forming isolated [MoO4]2– group. Owing to their similar ionic radius and valance state, the Eu3+ ion successfully replaces the Gd3+ ion and does not change the lattice site of the host Fe0.5Gd0.5(MoO4)1.5:Eu3+ and the crystal phase is very much pure. Further, no other extra peaks of impurity were observed.
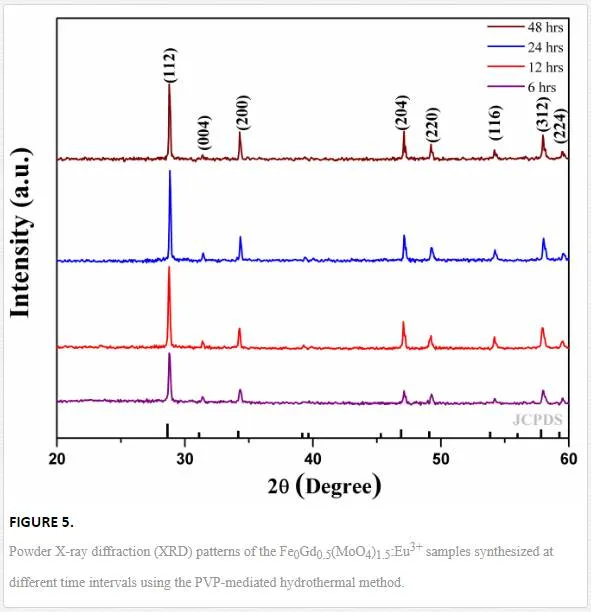
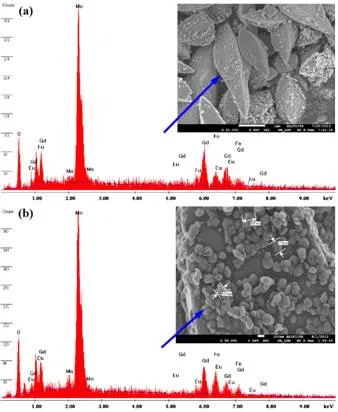
Figure 5 (a, b) shows the representative EDX analysis, taking single bipyramid-like particle and single nanoparticle sheathed on bipyramidal structure synthesized at 24 h time interval for Eu3+ doped Fe0.5Gd0.5(MoO4)1.5. The energy-dispersive X-ray spectrum was used to confirm the presence of iron (Fe3+), gadolinium (Gd3+), europium (Eu3+), molybdenum (Mo6+), and oxygen (O2–) in the final product. The EDX spectrum shown in Figure 5 (a, b) confirms the presence of Fe, Gd, Eu, Mo, and O in the final product.
OPTICAL AND MAGNETIC PROPERTIES OF FE0.5GD0.5(MOO4)1.5:EU3+ STRUCTURES
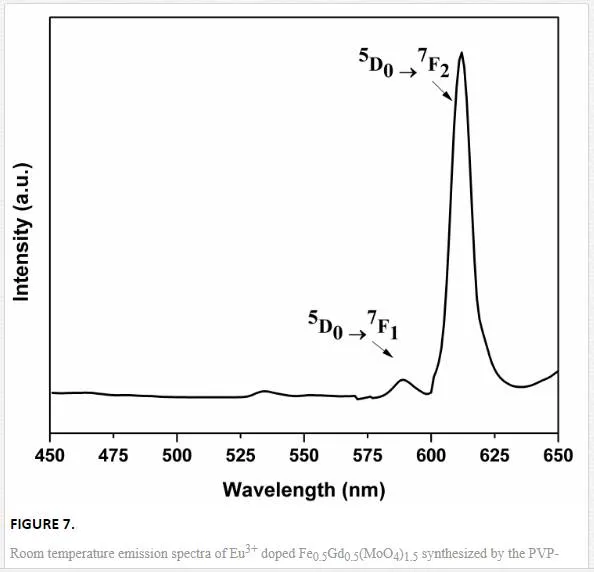
Figure 7 shows the PL excitation and emission spectra of nanoparticle sheathed bipyramid-like microstructures of Fe0.5Gd0.5(MoO4)1.5:Eu3+. Emission (λex = 395 nm) spectra of the Fe0.5Gd0.5(MoO4)1.5 doped with Eu3+ ions were recorded within the range from 575 to 700 nm at room temperature. Upon 395 nm UV irradiation, the emission spectrum was governed by the hypersensitive red emission, showing a transition 5D0→7F2 (due to electric dipole transition) higher than 5D0→7F1(magnetic dipole). In the emission spectrum, the transitions 5D0→7FJ (where J = 1, 2, 3, 4) were observed due to intra-configurational f–f electronic transitions of Eu3+ ions. The presence of electric dipole transition confirmed that Eu3+ ions were located at sites without inversion symmetry (C3vsymmetry). The other transitions 5D0→7F3 and 5D0→7F4 were comparatively very weak for all the samples (not shown in PL spectrum). However, the presence of magnetic iron (Fe3+) in the Fe0.5Gd0.5(MoO4)1.5 tetragonal crystal structure considerably decreases their luminescence, as Fe3+ions share the dodecahedral sites with Gd3+/Eu3+ [13]. However, it is still unclear for the weak red emission in Eu3+ doped Fe0.5Gd0.5(MoO4)1.5.
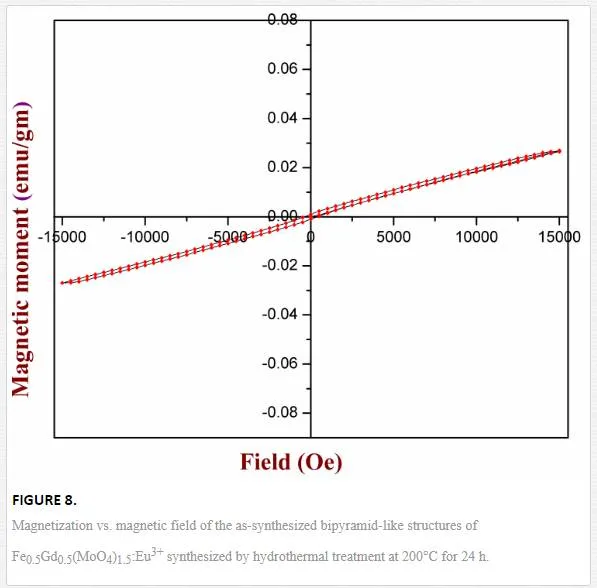
Figure 8 shows the room-temperature (RT) magnetic properties of hierarchically self-organized Fe0.5Gd0.5(MoO4)1.5:Eu3+ 3D microstructures synthesized by the hydrothermal route at 24 h time interval. Magnetization as a function of applied magnetic field (from 15000 Oe to 15000 Oe) was measured using a vibrating sample magnetometer. Due to the presence of Gd3+ ions, a magnetic hysteresis (M–H) loop was not observed in the case of Eu3+ doped Fe0.5Gd0.5(MoO4)1.5. From Figure 8, it is clear that a straight line crosses the origin, indicating that the phosphor Fe0.5Gd0.5(MoO4)1.5:Eu3+ exhibits a paramagnetic behavior due to the existence of paramagnetic Gd3+ions and the saturation magnetization (Ms) value is found to be 0.028 emu/gm for RT.
The extremely localized nature of the seven unpaired inner 4f electrons of Gd plays an important role which determines its magnetic properties [15]. These unpaired electrons in the outer orbital are closely bound to the nucleus and effectively shielded by the outer closed shell electrons 5s2 5p6 from the crystal field [15]. Even though the Gd3+ ions jointly occupy the dodecahedral sites with Fe3+ ions, number of 4f electrons in the Gd3+ ions, atomic unit cell volume, direct f–f exchange interactions between neighboring Gd3+ and Fe3+ atoms are responsible for setting up the magnetic moments [15, 14]. Since, Gd3+ ions have total orbital angular momentum L = 0 [13], the spin orbit coupling between the partially filled 4f electrons in the Gd3+ and Fe3+ ions is weak, which inhibits sufficient orbital overlaps [8].
Conclusion
In summary, the interaction between the Gd3+ and Fe3+ ions in the crystal structure Fe0.5Gd0.5(MoO4)1.5:Eu3+ is weak and hence insufficient overlap of the orbitals associated with 4f shells gives rise to paramagnetism. Further, more detailed investigations are required to determine the magnetic behavior of Fe0.5Gd0.5(MoO4)1.5:Eu3+ at various temperatures. The nanoparticle sheathed Fe0.5Gd0.5(MoO4)1.5:Eu3+ porous bipyramid-like structures were successfully synthesized using hydrothermal technique by employing PVP as the surfactant. The hydrothermal reaction time period was the key parameter and plays an important role for morphological evolution of self-aggregated 3D hierarchical networks. A possible growth mechanism for the formation of microstructures clearly involves the layer-by-layer self-assembly of 2D nanoflakes with successive disassembly, via an Ostwald ripening phenomena. The photoluminescence emission properties of Eu3+ doped Fe0.5Gd0.5(MoO4)1.5were investigated. Under the excitation of 395 nm UV light, the as-synthesized samples show red emission from the hypersensitive 5D0→7F2 transition at 615 nm. RT magnetic properties of the nanoparticles sheathed porous bipyramid-like Fe0.5Gd0.5(MoO4)1.5:Eu3+ structures clearly exhibit the paramagnetic behavior due to the presence of paramagnetic Gd3+ ions. The current experimental results are important and may open up a new scope for the design and formulation of bifunctional magnetic and luminescence applications.