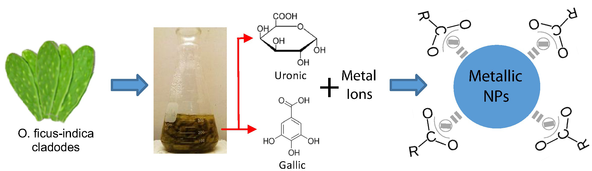
Introduction
Several nanoparticle synthesis methods are applied nowadays in different scientific fields; furthermore, based on the type of process (physical, chemical, or biological) and the conditions the synthesis is undertaken, they allow a control of the shape of the material, thus managing their application more accurately [1, 2]. With the objective of adopting eco-friendly methods that help to reduce the pollution caused by some toxic compounds and to exploit the local natural resources, the biosynthesis of nanostructured materials (green methods) has been undertaken through antioxidant microorganisms and agents obtained from local plant extracts [3–5]. Thus, the use of green synthesis in nanotechnology is fundamental in scientific research in nanoscience, mainly, in order to find medical applications of the nanostructures that reduce toxicity risks while being biocompatible [6–8]. There is evidence of nanoparticles obtained through size-tunable biosynthesis [9, 10] and different technological and biocompatible applications. For instance, Ag nanoparticles show antibacterial and antitumor properties and have potential use in antineoplastic treatments [11–14]; furthermore, they are applicable in SERS (surface-enhanced Raman spectroscopy) [15]. Gold nanoparticles between 20 and 25 nm present catalytic activity [16], and ZnO nanoparticles with a range between 9.6 and 25.5 nm present antibacterial and photocatalytic applications [17]. Pooja et al. synthesized biocompatible gold nanoparticles using karaya gum, which can be used in the elaboration of antineoplastic medications [18]. Biocompatible silver nanoparticles were obtained from the extract of Rosa damascen petals, which have anticarcinogenic properties against pulmonary adenocarcinoma [19]. Patra et al. synthesized gold and silver nanoparticles with Butea monosperma leaves; this nanoparticle system inhibits the growth of cancer cells, and these authors consider that the synthesis of these nanoparticles is important in biomedicine for the development of cancer treatments [20]. The biosynthesis of nanostructured systems thus appears to be a valuable tool for nano-biotechnological applications in nanoscience around the world.
Similarly, the green synthesis is used in the graphene and bimetallic nanoalloys obtained. Coconut water and pomegranate juice were reported as reducing and capping agents of graphite oxide (OG) to obtained graphene [21, 22]. Other authors have obtained Au-Ag bimetallic nanoparticles with pomegranate juice [23] and extract from leaf of mahogany [24], as well as the bioreduction synthesis in bimetallic nanostructure-type core/shell of Ti/Ni between 1 and 4 nm employing Medicago sativa [25]. Sheny and collaborators used plant extract of Anacardium occidentale for the formation of bimetallic nanoparticles of Au-Ag, considering that polyols play an important role in the reduction of metal ions [26].
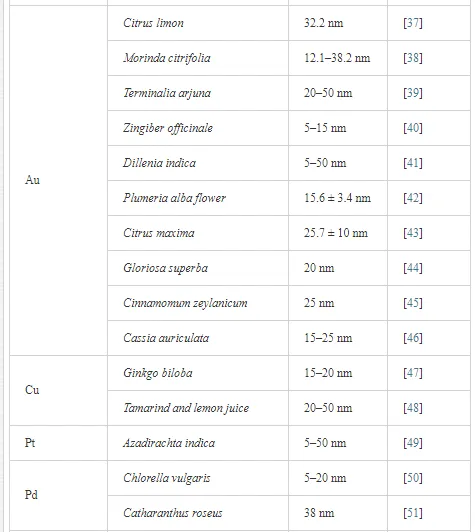
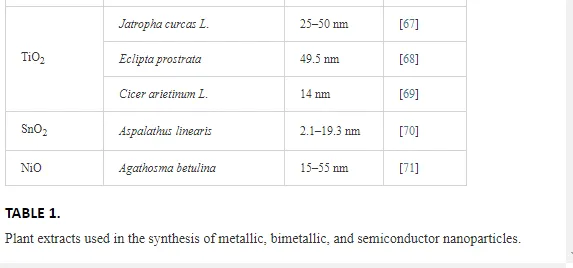
Table 1 lists a variety of plants that have been recently used for the synthesis of metallic, bimetallic, and semiconductor nanoparticles.

The role of carboxylic acids in nanoparticles
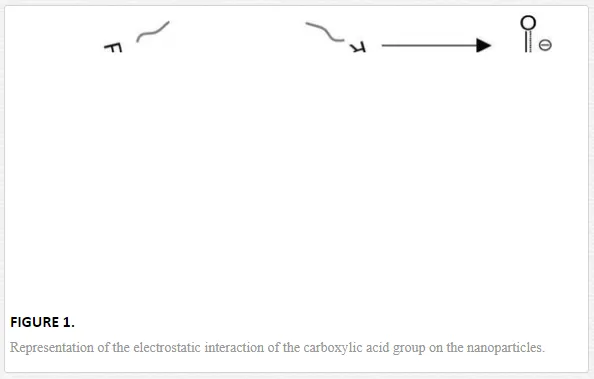
Nano-biosynthesis is classified as a chemical method that promotes the growth of a system by the aggregation of the metallic atoms reduced from the atoms in precursor solutions. Literature reports show that certain organic compounds are responsible for oxidation-reduction (redox) reactions, which trigger the formation and stabilization of nanoparticles. For instance, in the cases of the biometallic alloys Au, Ag, and Au/Ag, it is observed that polyphenols and polyoles that carry antioxidant properties present in plants have an important role in the formation of nanostructures [24, 72–74]. Quercetin (a flavonol with high antioxidant activity [75]) also appears in the reports as a main component in the formation of metallic nanostructures [76]. It is thus important to highlight the role that the antioxidant activity of the substances used in biosynthesis has on the fabrication of nanoparticles. Similarly, literature reports that carboxylic acids are commonly used in biological methods as reducing and sometimes stabilizing agents in the production and application of these materials. Yoosaf et al. show that it is possible to stabilize nanoparticles through electrostatic interactions with carboxylic groups (using gallic acid), which adhere to the surface of the nanoparticles [77]. This argument is supported by Amornkitbamrung et al. who attribute the reduction of Pd+2→Pd0 to the functionality of the carboxylate ion (R-COO-) [78]. On the other hand, Hosseini-M et al. address that carboxylic acids are crucial in the morphology, size, and distribution of Fe3O4 nanoparticles; furthermore, they present a co-catalyst effect [79]. Au nanoparticles were synthesized with dicarboxylic acids (oxalic, malonic, succinic, glutaric, and adipic) as reducing agents of HAuCl4, without the presence of any other surfactant agents, the synthesis resulted in different morphologies and SERS applications [80]. Similarly, other reports reiterate the importance of the carboxylic groups in the formation of nanoparticles [81, 82]. On nonmetals, Dwivedi et al. obtained selenium nanoparticles of 40–100 nm using carboxylic acids (acetic, oxalic, and gallic acids) for the synthesis method [83]. Propionic acid is used as a stabilizing agent in the fabrication of ZnO quantum dots (3.6–5.2 nm) [84]; similarly, carboxylic acids were used in manganese oxide nanoparticles, which work as catalysts in the conversion of CO to CO2 [85]; additionally, pimelic dicarboxylic acid is used as a nucleating agent for the synthesis of TiO2 nanoparticles [86]. Thus, the carboxylic acid groups stick to nanoparticles transferring stability (Figure 1), as reported by Zhi-Mei Qi et al. who synthesized gold nanoparticles through infrared (IR) spectroscopy [81].
Carboxylic acids are the most common type of organic acids in the carboxylic group (made by the fusion of one hydroxyl and carboxyl group) at the extreme end of the carbon chain. Under certain conditions, proton donors transfer H+ hydrons through heterolysis. The general formula of the carboxylic acid group is R-COOH, where R is a monovalent functional group (one hydrogen or carbon chain), when the carbon structure is replaced by two functional carboxylic groups, the acid is dicarboxylic acid (HOOC-R-COOH). When the proton H+ is transferred to the remaining ion, the formula changes into R-COO- carboxylate [87]. These acids are used in the food and pharmaceutical industries and in the manufacture of detergent and surfactant agents, among other applications [88]. Recent reports have demonstrated that when COOH groups are applied to certain biological complexes, they present excellent antitumor and antioxidant activity [89]; furthermore, other reports indicate that the carboxylic acids in Rhinacanthus nasutus show antiviral activity [90].
The use of these compounds in the synthesis processes of metallic and nonmetallic nanoparticles is increasing due to the biocompatible and antioxidant properties of carboxylic acids.
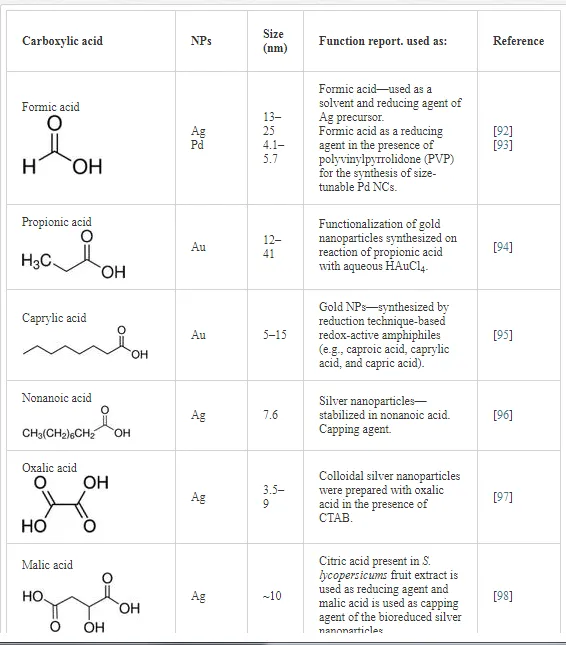
Table 2 shows scientific reports on carboxylic acids used in biosynthesis for the production of nanomaterials; the table focuses on the different applications of the acids. In regard to the reduction-oxidation process of metallic ions, Zoya Zaheer and Rafiuddin [91] propose a reduction mechanism of Ag+ by oxalic acid (HOOC-COO-) with CTAB as the stabilizing agent; the reduction mechanism takes place in an aqueous solution with a pH control of pK = 1.2 and pK2 = 4.2.
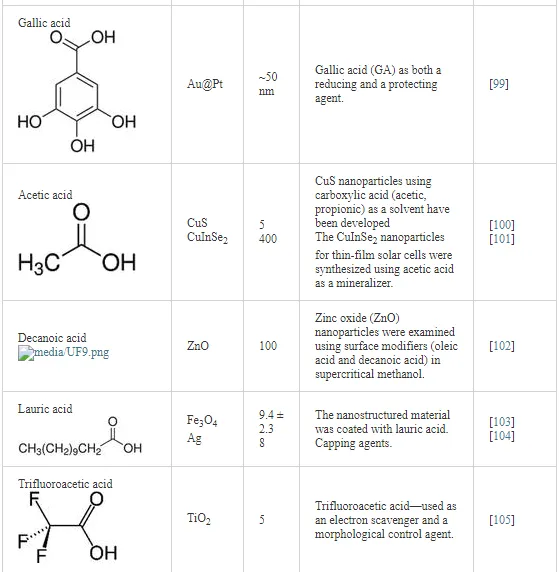

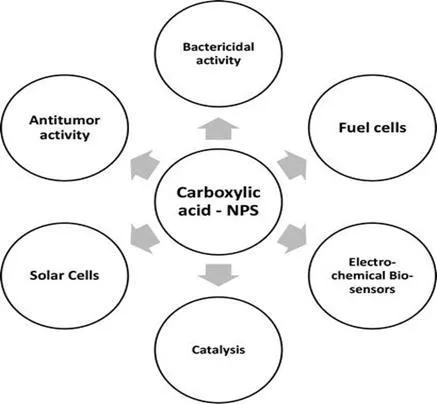
Since the organic compounds of carboxylic acids can be found in nature (for instance, in plants such as fungi) and they are not harmful for human consumption or the environment, they are used in the food and pharmaceutical industries. Studies on the synthesis of metallic and semiconductor nanoparticles are beginning to be used in these fields in order to improve processes that are beneficial to the environment and human beings. It is important to mention that some carboxylic acids functionalized with nanoparticles are also used in technological applications (Scheme 1).


A recent study shows a simpler unsaturated carboxylic acid (acrylic acid) that works with silver nanoparticles in the process of membrane filtration that avoids nanoparticles to adhere to the surface of the modified membranes, which at the same time show antibacterial/bacteriostatic properties [109]. The main reaction of acrylic acid is polymerization; thus, it is commonly used in the production of plastics, paints, and adhesives. Due to these characteristics, Ag nanoparticles modified with polyacrylic acid was produced by the redox method showing excellent water solubility, stability, and biocompatibility, as well as antibacterial properties against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [110]. Nanoparticles and formic acid have a similar role, since the electrochemical oxidation process of formic acid is an efficient energy supplier in direct formic acid fuel cells (DFAFCs) for mobile and portable applications [111–113]. The biodegradability and low cost of formic acid makes it a valuable resource in energy storage, in spite of its toxicity [114–117]. Thus, metallic and bimetallic nanoparticles Pt [118], Pt-Cu [119], Pt-Au [120, 121], Pd-Ag [122], and so on are used as catalysts in the electro-oxidation of formic acid.
Reports also show oxalic acid functionalized with biocompatible magnetite nanobars (oxalic acid-Fe3O4) prepared through the co-precipitation method for applications in electrochemical biosensors [123]. Additionally, oxalic acid and malic acid play an important role in the synthesis of tungstite nanoplates and nanoflowers (hydrated tungsten oxide: WO3⋅H2O) which show photocatalytic properties [124, 125]. Sedira et al. show that Ag nanoparticles oxidize easily in aqueous solutions, which in combination with acetic acid cause the liberation of Ag+ and improve the bactericidal effect in a higher range than with zinc oxide quantum dots [126]. Similarly, benzoic acid in the surface of TiO2 nanorods increases the power conversion efficiency of dye-sensitized solar cells (DSSCs), becoming at the same time an alternative and efficient method for the production of electrodes based on TiO2 nanorods [127]. It has been shown recently that the synthesis of Se nanoparticles induced by carboxylic acids (acetic acid, pyruvic acid, and benzoic acid) has antitumor properties with good potential in the treatment of Dalton’s lymphoma (DLA) cancer cells [128]. On the other hand, caffeic acid (containing the functional groups phenolic and acrylic) is used as a reducing and stabilizing agent in the preparation of silver nanoparticles, which show antitumor properties and works as an alternative agent in human hepatoma therapies [129]. Reports show that green synthesis of gold nanoparticles obtained with chlorogenic acid presents anti-inflammatory properties; additionally, it has promising applications in nanomedicine [130]. Maleic acid (dicarboxylic) functionalized with gold nanoparticles is used in the colorimetric detection of high efficiency of lead [131]; correspondingly, copper nanoparticles functionalized with carboxylic acid act as catalysts, when reducing 2-nitrophenol to 2-aminophenol in a few minutes [132]. Hence, it can be concluded that the different applications of metallic and nonmetallic nanoparticles in combination with organic agents such as carboxylic groups are of great importance in future medical applications for the development of antineoplastic therapies.
Biosynthesis of nanoparticles with Opuntia ficus-indica
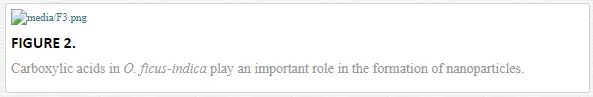
The cladodes from O. ficus-indica are characterized by their antioxidant properties, vitamin content, and by the presence of flavonoids and gallic acids [133–137], in addition to their content of uronic acid, a type of sugar acid with carbonyl and carboxylic functional groups [138]. In recent studies, gallic acid is used as a reducing and stabilizing agent in the mass production of silver nanoparticles with antioxidant properties and low cytotoxicity for normal cells [139, 140]. Similarly, biocompatible gold nanoparticles are synthesized at environment temperature through the reduction of HAuCl4 with gallic acid and poly-(N-vinyl-2-pyrrolidone) (PVP) [141]. Thus, carboxylic organic agents (gallic and uronic acids) in the cladode extract are responsible for the reduction and stabilization of the nanoparticles (Figure 2)
O. ficus-indica is well known for its anti-diarrheal, anti-inflammatory, antiviral, and anticarcinogen properties [142, 143], as well as for being used in treatments for diabetes and indigestion. The plant is commonly used as a nutritional complement, and its fruits and cladodes can be consumed in salads [144–146]. In general, this plant contains vitamins, minerals, and sugars, indispensable for human health. A recent study by E. Ramirez M et al. reports that O. ficus-indica cladodes improve the physicochemical properties of corn tortillas [147]; additionally, it increases the antioxidant activity in the blood and plasma of humans [148]. Furthermore, the extract has better anti-inflammatory potential than the drug indometacin [149]. Similarly, metallic and nonmetallic nanoparticles obtained with ecological methods are pioneering in the same fields as the extract O. ficus-indica (Scheme 2).

For instance, silver nanoparticles obtained from the extracts of R. indica and European black elderberryshow anti-inflammatory properties [150, 151]. Gold nanoparticles from Inonotus obliquus show antioxidant activity [152]. Synthesized ZnO nanoparticles from the root of Polygala tenuifolia show antioxidant and anti-inflammatory activity [153]. In the same way, Au and Ag nanoparticles obtained by biosynthesis are powerful nanomaterials in the control of diabetes [154–156]. Thus far, literature only reports in vitro studies with antibacterial activity using O. ficus-indica extract, applied in the biofabrication of silver nanoparticles, considering the synergic affects against E. coli and S. aureus[133]. As a consequence, the organic agents such as carboxylic acids, in the cladode extract of O. ficus-indica, and the nanoparticles obtained through eco-friendly methods, can improve the nanobiotechnological applications; furthermore, they secure innovative developments of the several application fields and the modern technology. It is still necessary to develop studies in order to validate the hypotheses presented in this study, concerning the nanoparticles obtained from the extracts from the cladodes.
Experimental section
METALLIC NANOPARTICLES
In this study, we synthesized metallic nanoparticles Ag, Au, Cd, Cu, Pb, and Ti with cladodes from O. ficus-indica in a colloidal medium. The synthesis presented excellent stability during long periods of time. The following were used as precursors during the synthesis processes: Nitrates AgNO3; Cu(NO3)2; Pb(NO3)2; Cd(NO3)2; Chlorides: HAuCl4. Small fragments of metal underwent a thermal treatment in nitric acid for the synthesis of Ti nanoparticles (Figure 2). The method used in this study was made in collaboration with other authors [157]: 25 g of the cladode was mixed in 50 ml of deionized water; subsequently, the solution underwent thermal treatment at a constant temperature of 60°C, and magnetic agitation for 1 h. The resulting solution is then filtered obtaining the O. ficus-indicaextract. Three milliliters of the extract is mixed with 25 ml of the precursor solutions (nitrate and chlorides) for the reduction of the metallic ions. The solution undergoes the same thermal treatment and magnetic agitation described above. The nanoparticles are formed and stabilized during these processes.
CARBON NANOSTRUCTURES
The organic molecules containing the O. ficus-indica extract may have hydrophilic properties. This represents of the extract of the plant a strong candidate for the obtaining of laminar materials of carbon and small quantum dots both in colloidal means.
To obtain a few graphene sheets from commercial graphite, 5 ml of the extract of O. ficus-indica was used as mentioned in the previous section. Subsequently, 2 g of commercial graphite was added to 50 ml of deionized water and mixed with 5-ml extract of O. ficus-indica. The mixture was kept in an ultrasonic bath for 30 min. Finally, floating material was collected on the liquid surface with a slightly bright hue, to be analyzed by transmission electron microscopy (TEM), Raman, and X-ray photoelectron spectroscopy (XPS). To obtain carbon quantum dots (CQDs), a mixture with the same components was maintained under magnetic stirring and at 50°C for 30 min and then subjected to an ultrasonic bath for 30 min. In the first minutes of subjecting the sample in the ultrasonic bath, a tone change in the surface of the liquid is observed. In the same way, floating material was collected on the surface of the liquid, finding small CQD.
Results and discussio
Nanoparticles of the precursors ns mentioned in the experimental section were obtained and characterized by transmission electron microscopy. In the case of the silver nanoparticles, these mainly presented particle sizes that oscillate between 2 and 4 nm, and few cases are observed with sizes between 10 and 15 nm (Figure 3a). In both cases, morphologies of spherical type are observed. For the case of the precursor HAuCl4 after performing the synthesis process mentioned in the experimental section, gold nanoparticles with different morphologies such as triangular, pentagonal, hexagonal, and quasi-spherical were obtained. The mentioned macroscopic parameters allowed a diversity of morphologies for this precursor as seen in Figure 3b. In the case of cadmium nanoparticles (Figure 3c), an irregular shape with sizes located between 2 and 8 nm approximately was obtained.

In some cases, the organic molecules contained in the plant extract are manifested by interacting with the surface of the nanoparticle, possibly as a consequence of molecular affinity. As is the case with copper nanoparticles (Figure 3d), in these, a region between 2 and 3 nm in thickness is observed that surrounds a nanoparticle with a diameter of approximately 10 nm. Another similar case was presented when synthesizing titanium nanoparticles; in the TEM image (Figure 3f), we observed clusters of nanoparticles were stabilized by an organic medium. The nanoparticles of titanium have sizes located at approximately 5 nm; we assume that this stabilizing medium may contain ascorbic acid, starches, proteins, and various vitamins naturally contained in the extract of the plant O. ficus-indica.
On the other hand, when using PbNO3 as a precursor of lead nanoparticles by the synthesis method presented, nanoparticles below 10 nm were obtained with well-defined crystalline phase as seen in Figure 3e. For the case of the synthesized metallic nanoparticles, we observed that the extract of O. ficus-indica facilitates the obtaining for a size smaller than 10 nm. This has several advantages for analyzing biomedical applications such as drug delivery, therapeutic applications, bioimaging, and magnetic energy storage [158–160].
For the laminar carbon nanostructures obtained by green synthesis methods, there are currently published results that start from graphite oxide as a precursor [161, 162]. In the present investigation, we use commercial graphite as a precursor, further reducing the costs of synthesis for the nanostructured laminar final product. Figure 3g and h show graphene layers made up of less than 10 layers. These were obtained by the simple ultrasonic sonication method shown in the experimental section. We assume that the method presented can be made repeatedly until a smaller number of graphene sheets are obtained because the hydrophilic components in the extract of O. ficus-indica favor the exfoliation.
The use of green synthesis to obtain CQD is rarely documented. Few numbers of articles show evidence of the synthesis of CQD using plant extract [163, 164]. By combining the O. ficus-indica plant extract with a small amount of commercial graphite and maintaining the mixture at 60° C for 1 h, it was possible to collect the surface liquid from the solution for further analysis by TEM and to find CQD (Figure 3i). In the same way as the metallic nanoparticles, the extract allowed to obtain CQD with a size smaller than 5 nm. This favors the applications of chemical sensors, photodetectors, and so on [165, 166].
Optical properties in metallic nanoparticles
As is well known, the metallic and semiconductor nanoparticles have new optical properties in relation to the bulk material. These properties can be detected by ultraviolet/visible spectroscopy (UV/Vis) and are associated with the existence of surface plasmon. Surface plasmon resonance (SPR) physically represents the oscillation of free electrons on the surface of the nanoparticles, constituting a characteristic fingerprint of each nanostructured material. The nanoparticles obtained using the extract of O. ficus-indica were analyzed experimentally by UV/Vis spectroscopy. All spectra were considered from 200 to 800 nm.
In the case of silver nanoparticles, these showed an absorption band centered on 390-nm characteristic of the SPR due to quantum confinement in silver, as seen in Figure 4a. The dependence of the location of the SPR is associated with the morphology of the nanostructures as well as the size. Silver nanostructures can be found in the literature with an SPR located at 408, 430, 440, and so on [167–169], associated with different sizes of silver nanoparticles.
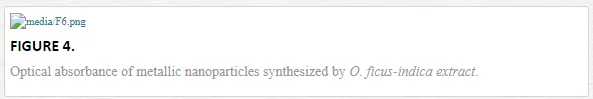
The gold nanoparticles probably represent the most studied metal nanostructured in terms of behavior and shifts of SPR. The gold nanoparticles have absorption bands located at 500, 550, 800, and so on, for nanoparticles with different morphologies. This indicates the dependence and sensitivity of the location of the SPR with the morphology of the gold nanostructures [170–172]. The gold nanoparticles obtained in this work show a large amplitude absorption band associated with the presence of the SPR centered at 55 nm. We assume that this band implicitly considers the contribution of several SPRs associated with the different morphologies obtained, as shown in Figure 4b. This can be seen in the large amplitude of the absorption band, with a range from 500 to 670 nm. For cadmium nanoparticles, an absorption band centered at 232 nm is shown in Figure 4c. For lead, there are reports of nanocubes with absorption bands located at approximately 320 and 400 nm [173]. A band detected at 300 nm was associated with SPR due to the confinement in these lead nanoparticles (Figure 4e).
The obtained copper nanoparticles had a well-defined absorption band centered at 580 nm approximately as shown in Figure 4d. The synthesis of copper nanoparticles it faces to copper oxidation easily in a colloidal medium, the extract of O. ficus-indica facilitates the stabilization of these nanoparticles. Prabsash et al. obtained nanoparticles of copper with a size of 10 nm, using chemical reduction by the reducing agent sodium borohydride [174].
On the other hand, titanium (metal) nanoparticles are difficult to find in the literature. There are reports from Mohammadi and Halali who used the electromagnetic levitation melting gas method to evaporate titanium particles [175]; the nanoparticles obtained by them have sizes between 28 and 40 nm. Although the method presented is effective, it represents a requirement of special equipment to carry out the synthesis of titanium nanoparticles. The titanium nanoparticles obtained in the present work were based on the modified method presented by R. Britto et al. [176], varying slightly the amount of extract of the plant O. ficus-indica. The absorption band obtained for titanium nanoparticles (Figure 4f) was located at 350 nm, associated with the SPR of this metal.
Conclusions
In conclusion, the current importance of organic agents functionalized with nanoparticles in the nanotechnological and biomedical fields has been exposed in this study. The properties of carboxylic acids make the fabrication of biocompatible nanostructured systems attractive for future attention. The biosynthesis process used in the fabrication of graphene layers and nanoparticles initiates an ecological and low-cost alternative in biocompatible applications for the treatment of diseases, mainly for antineoplastic therapies. The cladode extract from O. ficus-indica is highly efficient in the formation of nanoparticles, which can play an important role in the biocompatibility for the benefit of health and nutrition. Metallic and nonmetallic nanoparticles with nanobiotechnological applications synthesized with ecological methods (carboxylic groups) will be a fundamental tool for biocompatible applications in nanoscience and nanotechnology.