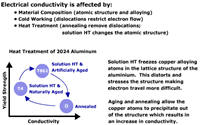
With some materials, such as solution heat treatable aluminum alloys, conductivity measurements are often made verifying that parts and materials have received the proper heat treatment. High purity aluminum is soft and ductile, and gains strength and hardness with the addition of alloying elements. A few such aluminum alloys are the 2000 series (2014, 2024, etc.), 6000 series (6061, 6063, etc.), and 7000 series (7050, 7075, etc.). The 2xxx series aluminum alloys have copper, the 6xxx series have magnesium, and the 7xxx have zinc as their major alloying elements.
Heat treatment of aluminum alloys is accomplished in two phases – solution heat treatment and then aging. In the solution heat treatment step, the alloys are heated to an elevated temperature to dissolve the alloying elements into solution. The metal is then rapidly cooled or quenched to “freeze” the atoms of the alloying elements in the lattice structure of the aluminum. This distorts and stresses the structure, making electron movement more difficult, thereby decreasing the electrical conductivity. In this condition, the alloys are still relatively soft but start to gain strength as the alloying elements begin to precipitate out of solution to form extremely small particles that impede the movement of dislocations within the material. The formation of the precipitates can be controlled for many alloys by heating and holding the material at an elevated temperature for a period of time (artificial aging). As the alloying elements precipitate out of solid solution, the conductivity of the material gradually increases. By controlling the amount of precipitated particles within the aluminum, the properties can be controlled to produce peak strength or some combinations of strength and corrosion resistance. Sometimes, the material must be annealed or put into the softest, most ductile condition possible in order to perform forming operations. Annealing allows all of the alloying elements to precipitate out of solution to form a coarse, widely spaced precipitate. The electrical conductivity is greatest when the material is in the annealed condition.
Since solution heat-treated and aged materials are stronger, components can be made using less material. A lighter or more compact design is often of great importance to the designer and well worth the cost of the heat treating process. However, think of the consequences that could arise if a component that was supposed to be solution heat-treated and aged somehow left the manufacturing facility and was put into service unheat-treated or annealed. This is a real possibility since heat-treated aluminum parts look exactly like unheat-treated parts. Consider 2024 aluminum as an example. Select tensile properties and its electrical conductivity for various heat treatment conditions are given in the following table.
Properties for Alclad 2024 Aluminum
| Heat Treatment Condition | Ultimate Strength | Yield Strength | Electrical Conductivity |
| Annealed (O) | 26 ksi (180 MPa) | 11 ksi (75 MPa) | 50 % IACS |
| Solution Heat Treated and Naturally Aged (T42) | 64 ksi (440 MPa) | 42 ksi (290 MPa) | 30 % IACS |
| Solution Heat Treated, Coldworked and Artificially Aged (T861) | 70 ksi (485 MPa) | 66 ksi (455 MPa) | 38 % IACS |
It can be seen that the yield strength for the material is 42 kilopounds/square inch (ksi) (290 MPa) in the solution heat-treated and naturally aged condition (T42 condition). The yield strength can be increased to 66 ksi (455 MPa) when coldworked and artificially aged (T861 condition). But in the annealed condition, the yield strength is reduced to 11 ksi (75 MPa). If an annealed part were accidentally used where a part in the T42 or T861 was intended, it would likely fail prematurely. However, a quick check of the conductivity using an eddy current instrument of all parts prior to shipping would prevent this from occurring.