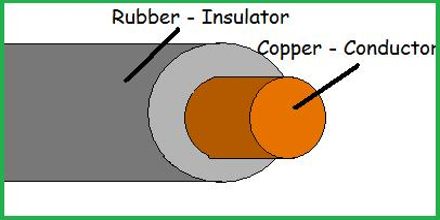
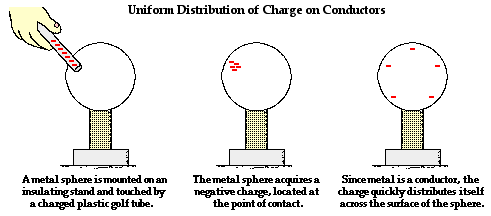
The behavior of an object that has been charged is dependent upon whether the object is made of a conductive or a nonconductive material. Conductors are materials that permit electrons to flow freely from particle to particle. An object made of a conducting material will permit charge to be transferred across the entire surface of the object. If charge is transferred to the object at a given location, that charge is quickly distributed across the entire surface of the object. The distribution of charge is the result of electron movement. Since conductors allow for electrons to be transported from particle to particle, a charged object will always distribute its charge until the overall repulsive forces between excess electrons is minimized. If a charged conductor is touched to another object, the conductor can even transfer its charge to that object. The transfer of charge between objects occurs more readily if the second object is made of a conducting material. Conductors allow for charge transfer through the free movement of electrons.
In contrast to conductors, insulators are materials that impede the free flow of electrons from atom to atom and molecule to molecule. If charge is transferred to an insulator at a given location, the excess charge will remain at the initial location of charging. The particles of the insulator do not permit the free flow of electrons; subsequently charge is seldom distributed evenly across the surface of an insulator.
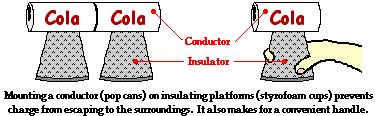
While insulators are not useful for transferring charge, they do serve a critical role in electrostatic experiments and demonstrations. Conductive objects are often mounted upon insulating objects. This arrangement of a conductor on top of an insulator prevents charge from being transferred from the conductive object to its surroundings. This arrangement also allows for a student (or teacher) to manipulate a conducting object without touching it. The insulator serves as a handle for moving the conductor around on top of a lab table. If charging experiments are performed with aluminum pop cans, then the cans should be mounted on top of Styrofoam cups. The cups serve as insulators, preventing the pop cans from discharging their charge. The cups also serve as handles when it becomes necessary to move the cans around on the table.
Examples of Conductors and Insulators
Examples of conductors include metals, aqueous solutions of salts (i.e., ionic compounds dissolved in water), graphite, and the human body. Examples of insulators include plastics, Styrofoam, paper, rubber, glass and dry air. The division of materials into the categories of conductors and insulators is a somewhat artificial division. It is more appropriate to think of materials as being placed somewhere along a continuum. Those materials that are super conductive (known as superconductors) would be placed at on end and the least conductive materials (best insulators) would be placed at the other end. Metals would be placed near the most conductive end and glass would be placed on the opposite end of the continuum. The conductivity of a metal might be as much as a million trillion times greater than that of glass.

Along the continuum of conductors and insulators, one might find the human body somewhere towards the conducting side of the middle. When  the body acquires a static charge it has a tendency to distribute that charge throughout the surface of the body. Given the size of the human body, relative to the size of typical objects used in electrostatic experiments, it would require an abnormally large quantity of excess charge before its effect is noticeable. The effects of excess charge on the body are often demonstrated using a Van de Graaff generator. When a student places their hand upon the static ball, excess charge from the ball is shared with the human body. Being a conductor, the excess charge could flow to the human body and spread throughout the surface of the body, even onto strands of hair. As the individual strands of hair become charged, they begin to repel each other. Looking to distance themselves from their like-charged neighbors, the strands of hair begin to rise upward and outward – a truly hair-raising experience.
the body acquires a static charge it has a tendency to distribute that charge throughout the surface of the body. Given the size of the human body, relative to the size of typical objects used in electrostatic experiments, it would require an abnormally large quantity of excess charge before its effect is noticeable. The effects of excess charge on the body are often demonstrated using a Van de Graaff generator. When a student places their hand upon the static ball, excess charge from the ball is shared with the human body. Being a conductor, the excess charge could flow to the human body and spread throughout the surface of the body, even onto strands of hair. As the individual strands of hair become charged, they begin to repel each other. Looking to distance themselves from their like-charged neighbors, the strands of hair begin to rise upward and outward – a truly hair-raising experience.
Many are familiar with the impact that humidity can have upon static charge buildups. You have likely noticed that bad hair days, doorknob shocks and static clothing are most common during winter months. Winter months tend to be the driest months of the year with humidity levels in the air dropping to lower values. Water has a tendency to gradually remove excess charge from objects. When the humidity is high, a person acquiring an excess charge will tend to lose that charge to water molecules in the surrounding air. On the other hand, dry air conditions are more conducive to the buildup of static charge and more frequent electric shocks. Since humidity levels tend to vary from day to day and season to season, it is expected that electrical effects (and even the success of electrostatic demonstrations) can vary from day to day.
Distribution of Charge via Electron Movement
Predicting the direction that electrons would move within a conducting material is a simple application of the two fundamental rules of charge interaction. Opposites attract and likes repel. Suppose that some method is used to impart a negative charge to an object at a given location. At the location where the charge is imparted, there is an excess of electrons. That is, the multitude of atoms in that region possess more electrons than protons. Of course, there are a number of electrons that could be thought of as beingquite contented since there is an accompanying positively charged proton to satisfy their attraction for an opposite. However, the so-called excess electrons have a repulsive response to each other and would prefer more space. Electrons, like human beings, wish to manipulate their surroundings in an effort to reduce repulsive effects. Since these excess electrons are present in a conductor, there is little hindrance to their ability to migrate to other parts of the object. And that is exactly what they do. In an effort to reduce the overall repulsive effects within the object, there is a mass migration of excess electrons throughout the entire surface of the object. Excess electrons migrate to distance themselves from their repulsive neighbors. In this sense, it is said that excess negative charge distributes itself throughout the surface of the conductor.
But what happens if the conductor acquires an excess of positive charge? What if electrons are removed from a conductor at a given location, giving the object an overall positive charge? If protons cannot move, then how can the excess of positive charge distribute itself across the surface of the material? While the answers to these questions are not as obvious, it still involves a rather simple explanation that once again relies on the two fundamental rules of charge interaction. Opposites attract and likes repel. Suppose that a conducting metal sphere is charged on its left side and imparted an excess of positive charge. (Of course, this requires that electrons be removed from the object at the location of charging.) A multitude of atoms in the region where the charging occurs have lost one or more electrons and have an excess of protons. The imbalance of charge within these atoms creates effects that can be thought of as disturbing the balance of charge within the entire object. The presence of these excess protons in a given location draws electrons from other atoms. Electrons in other parts of the object can be thought of as being quite contented with the balance of charge that they are experiencing. Yet there will always be some electrons that will feel the attraction for the excess protons some distance away. In human terms, we might say these electrons are drawn by curiosity or by the belief that the grass is greener on the other side of the fence. In the language of electrostatics, we simply assert that opposites attract – the excess protons and both the neighboring and distant electrons attract each other. The protons cannot do anything about this attraction since they are bound within the nucleus of their own atoms. Yet, electrons are loosely bound within atoms; and being present in a conductor, they are free to move. These electrons make the move for the excess protons, leaving their own atoms with their own excess of positive charge. This electron migration happens across the entire surface of the object, until the overall sum of repulsive effects between electrons across the whole surface of the object are minimized.
Check Your Understanding
Use your understanding of charge to answer the following questions. When finished, click the button to view the answers.
1. One of these isolated charged spheres is copper and the other is rubber. The diagram below depicts the distribution of excess negative charge over the surface of two spheres. Label which is which and support your answer with an explanation.

See Answer

Answer: A is rubber and B is copper.
Sphere A shown a non-uniform distribution of excess charge; so sphere A must be made of an insulating material such as rubber. Sphere B shows a uniform distribution of excess charge; one would reason that it is made of a conductor such as copper.
close
2. Which of the following materials are likely to exhibit more conductive properties than insulating properties? _____ Explain your answers.
| a. rubber | b. aluminum | c. silver | d. plastic | e. wet skin |
See Answer

Answer; B, C and E
Aluminum and silver are metals, making them good conductors. The human body is a fairly good conductor. When wet, its an even better conductor.
3. A conductor differs from an insulator in that a conductor ________.
a. has an excess of protons
b. has an excess of electrons
c. can become charged and an insulator cannot
d. has faster moving molecules
e. does not have any neutrons to get in the way of electron flow
f. none of these
See Answer

Answer: F
A and B are characteristic of positive and negative objects. As for C, both insulators and conductors can be charged. As for D, this has nothing to do with the conductive properties of materials. As for E, neutrons are located in the nucleus and are “out of the way” of mobile electrons.
4. Suppose that a conducting sphere is charged positively by some method. The charge is initially deposited on the left side of the sphere. Yet because the object is conductive, the charge spreads uniformly throughout the surface of the sphere. The uniform distribution of charge is explained by the fact that ____.
a. the charged atoms at the location of charge move throughout the surface of the sphere
b. the excess protons move from the location of charge to the rest of the sphere
c. excess electrons from the rest of the sphere are attracted towards the excess protons
See Answer

Answer: C
Rule out A since atoms are not capable of moving within solid spheres. Rule out B since protons are not capable of moving in electrostatic demos. C is the proper explanation since the negative electrons are attracted to the region of positive charge. The electrons migrate towards the left side of the sphere until there is a uniform distribution of positive charge.
5. When an oil tanker car has arrived at its destination, it prepares to empty its fuel into a reservoir or tank. Part of the preparation involves connecting the body of the tanker car with a metal wire to the ground. Suggest a reason for why is this done.
See Answer

As fuel is pumped from the tanker car to a reservoir, charge can quickly build up as the fluid flows through the hoses. This static charge can create sparks capable of igniting the fuel. By connecting the body of the tanker car to the ground, the static charge can be transferred to the ground. A metal wire is used since metals are conductive and allow charge to flow through them.