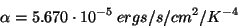A blackbody refers to an opaque object that emits thermal radiation. A perfect blackbody is one that absorbs all incoming light and does not reflect any. At room temperature, such an object would appear to be perfectly black (hence the term blackbody). However, if heated to a high temperature, a blackbody will begin to glow with thermal radiation.
In fact, all objects emit thermal radiation (as long as their temperature is above Absolute Zero, or -273.15 degrees Celsius), but no object emits thermal radiation perfectly; rather, they are better at emitting/absorbing some wavelengths of light than others. These uneven efficiencies make it difficult to study the interaction of light, heat and matter using normal objects.
Fortunately, it is possible to construct a nearly-perfect blackbody. Construct a box made of a thermally conductive material, such as metal. The box should be completely closed on all sides, so that the inside forms a cavity that does not receive light from the surroundings. Then, make a small hole somewhere on the box. The light coming out of this hole will almost perfectly resemble the light from an ideal blackbody, for the temperature of the air inside the box.
At the beginning of the 20th century, scientists Lord Rayleigh, and Max Planck (among others) studied the blackbody radiation using such a device. After much work, Planck was able to empirically describe the intensity of light emitted by a blackbody as a function of wavelength. Furthermore, he was able to describe how this spectrum would change as the temperature changed. Planck’s work on blackbody radiation is one of the areas of physics that led to the foundation of the wonderful science of Quantum Mechanics, but that is unfortunately beyond the scope of this article.
What Planck and the others found was that as the temperature of a blackbody increases, the total amount of light emitted per second increases, and the wavelength of the spectrum’s peak shifts to bluer colors (see Figure 1).
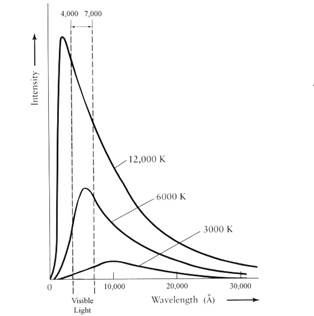
Figure 1
For example, an iron bar becomes orange-red when heated to high temperatures and its color progressively shifts toward blue and white as it is heated further.
In 1893, German physicist Wilhelm Wien quantified the relationship between blackbody temperature and the wavelength of the spectral peak with the following equation:
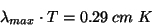
where T is the temperature in Kelvin. Wien’s law (also known as Wien’s displacement law) states that the wavelength of maximum emission from a blackbody is inversely proportional to its temperature. This makes sense; shorter-wavelength (higher-frequency) light corresponds to higher-energy photons, which you would expect from a higher-temperature object.
For example, the sun has an average temperature of 5800 K, so its wavelength of maximum emission is given by:
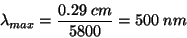
This wavelengths falls in the green region of the visible light spectrum, but the sun’s continuum radiates photons both longer and shorter than lambda(max) and the human eyes perceives the sun’s color as yellow/white.
In 1879, Austrian physicist Stephan Josef Stefan showed that the luminosity, L, of a black body is proportional to the 4th power of its temperature T.
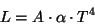
where A is the surface area, alpha is a constant of proportionality, and T is the temperature in Kelvin. That is, if we double the temperature (e.g. 1000 K to 2000 K) then the total energy radiated from a blackbody increase by a factor of 24 or 16.
Five years later, Austrian physicist Ludwig Boltzman derived the same equation and is now known as the Stefan-Boltzman law. If we assume a spherical star with radius R, then the luminosity of such a star is
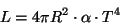
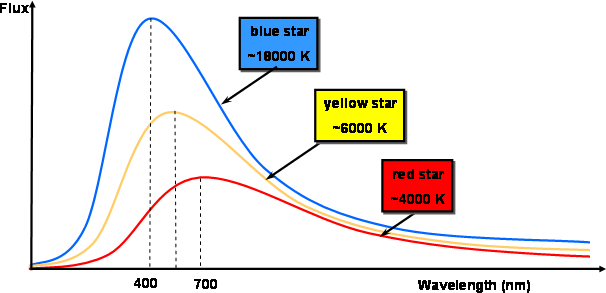
where R is the star radius in cm, and the alpha is the Stefan-Boltzman constant, which has the value: