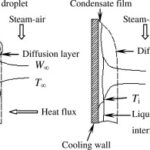
1) A heat pipe is a simple device with no moving parts that can transfer large quantities of heat over fairly large distances essentially at a constant temperature without requiring any power input.
2) It is composed of three sections:
a) Evaporator section at one end, where heat is absorbed and the fluid is vaporized
b) Condenser section at the other end, where the vapor is condensed and heat is rejected
c) Adiabatic section in between, where the vapor and the liquid phases of the fluid flow in opposite directions through the core and the wick to complete the cycle with no significant heat transfer between the fluid and the surrounding medium.
3) The Operation of a Heat Pipe
a) At a specified pressure, a liquid will vaporize or a vapor will condense at a certain temperature, called the saturation temperature. Thus, fixing the pressure inside a heat pipe fixes the temperature at which phase change will occur.
b) At a specified pressure or temperature, the amount of heat absorbed as a unit mass of liquid vaporizes is equal to the amount of heat rejected as that vapor condenses.
c) The capillary pressure developed in a wick will move a liquid in the wick even against the gravitational field as a result of the capillary effect.
d) A fluid in a channel flows in the direction of decreasing pressure.