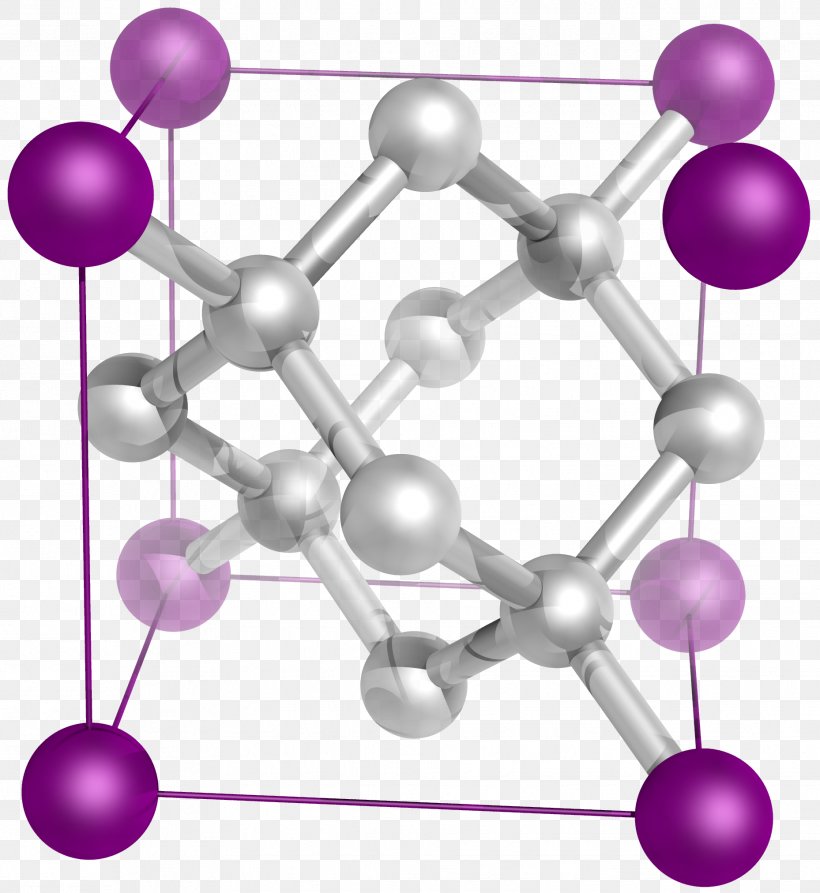
The seven crystal systems are a method of classifying crystals according to their atomic lattice or structure. The atomic lattice is a three dimensional network of atoms that are arranged in a symmetrical pattern. The shape of the lattice determines not only which crystal system the stone belongs to, but all of its physical properties and appearance. In some crystal healing practices the axial symmetry of a crystal is believed to directly influence its metaphysical properties. For example crystals in the Cubic System are believed to be grounding, because the cube is a symbol of the element Earth.
There are seven crystal systems or groups, each of which has a distinct atomic lattice. Here we have outlined the basic atomic structure of the seven systems, along with some common examples of each system.
Cubic System
Also known as the isometric system. All three axes are of equal length and intersect at right angles. Based on a square inner structure.
Crystal shapes include:
- Cube (diamond, fluorite, pyrite)
- Octahedron (diamond, fluorite, magnetite)
- Rhombic dodecahedron (garnet, lapis lazuli rarely crystallises)
- Icosi-tetrahedron (pyrite, sphalerite)
- Hexacisochedron (pyrite).
Common Cubic Crystals:
| Diamond | Fluorite | Garnet |
| Gold | Pyrite | Silver |
| Spinel |
Tetragonal System
Two axes are of equal length and are in the same plane, the main axis is either longer or shorter, and all three intersect at right angles.
Based on a rectangular inner structure.
Crystal shapes include:
- Four-sided prisms and pyramids
- Trapezohedrons
- Eight-sided and double pyramids
- Icosi-tetrahedron (pyrite, sphalerite)
- Hexacisochedron (pyrite).
Common Tetragonal Crystals:
| Anatase | Apophyllite | Chalcopyrite |
| Rutile | Scapolite | Scheelite |
| Wulfenite | Zircon |
Hexagonal System
Three out of the four axes are in one plane, of the same length, and intersect each other at angles of 60 degrees. The fourth axis is of a different length and intersects the others at right angles.
Based on a hexagonal (6-sided) inner structure.
Crystal shapes include:
- Four-sided prisms and pyramids
- Twelve-sided pyramids
- Double pyramids
Common Hexagonal Crystals:
| Apatite | Aquamarine | Beryl |
| Cancrinite | Emerald | Goshenite |
| Morganite | Sugilite | Zincite |
Trigonal System
(Rhombohedral System) – Axes and angles in this system are similar to the Hexagonal System, and the two systems are often combined as Hexagonal. In the cross-section of a Hexagonal crystal, there will be six sides. In the cross-section of a Trigonal crystal there will be three sides.
Based on a triangular inner structure.
Crystal shapes include:
- Three-sided prisms or pyramids
- Rhombohedra
- Scalenohedra
Common Trigonal Crystals:
| Agate | Amethyst | Aventurine |
| Calcite | Carnelian | Citrine |
| Hematite | Jasper | Phenakite |
| Quartz | Rhodochrosite | Rose Quartz (rarely crystallises) |
| Ruby | Sapphire | Smoky Quartz |
| Tigers Eye | Tourmaline |
Orthorhombic System
(Rhombic System)Three axes, all of different lengths, are at right angles to each other.
Based on a rhombic (diamond-shaped) inner structure.
Crystal shapes include:
- Pinacoids
- Rhombic prisms
- Pyramids
- Double pyramids
Common Orthorhombic Crystals:
| Alexandrite | Andalusite (Chiastolite) | Celestite |
| Chrysoberyl | Chrysoberyl | Danburite |
| Dumortierite | Enstatite | Hemimorphite |
| Iolite | Tanzanite | Topaz |
| Zoisite |
Monoclinic System
There are three axes, each of different lengths. Two are at right angles to each other and the third is inclined.
Based on a parallelogram inner structure.
Crystal shapes include:
- Basal pinacoids and prisms with inclined end faces
Common Monoclinic Crystals:
| Azurite | Chrysocolla | Diopside |
| Epidote | Gypsum | Hiddenite |
| Howlite | Kunzite | Lazulite |
| Moonstone | Muscovite (Mica) | Petalite |
| Serpentine | Spodumene | Staurolite |
| Vivianite |
Triclinic System
All three axes are of different lengths and inclined towards each other.
Based on a ‘triclinic’ inner structure, meaning ‘three inclined angles’.
Crystal forms are usually paired faces.
Common Triclinic Crystals:
| Amazonite | Aventurine Feldspar | Kyanite |
| Labradorite | Rhodonite | Turquoise |
Amorphous
No crystal structure. Most of these are either cooled too quickly to crystallise – such as obsidian or moldavite, or are organic – such as amber.
Common Amorphous Minerals:
| Amber | Moldavite | Obsidian |
| Opal |