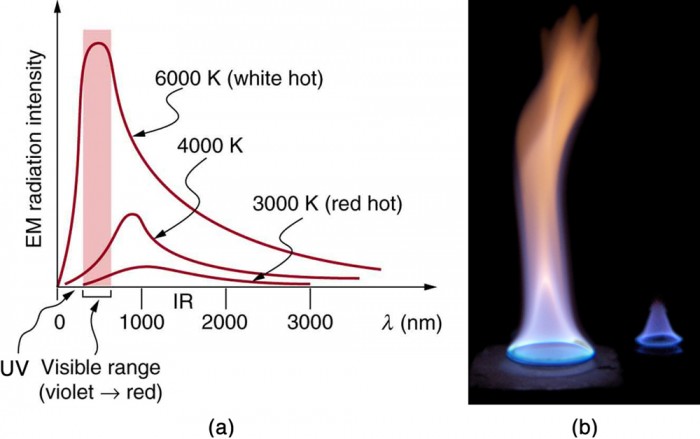
1) Gases that consist of monatomic molecules such as Ar and He and symmetric diatomic molecules such as N2 and O2 are essentially transparent to radiation, except at extremely high temperatures at which ionization occurs.
2) Gases with asymmetric molecules such as H2O, CO2, CO, SO2, and hydrocarbons HnCm may participate in the radiation process by absorption at moderate temperatures, and by absorption and emission at high temperatures such as those encountered in combustion chambers.
3) The presence of a participating medium complicates the radiation analysis considerably for several reasons:
a) Absorption and emission of participating medium is volumetric phenomenon
b) Absorption and emission only in narrow wavelength band i.e. gray assumption is not always valid
c) Absorption and emission also depends on constituents gas of mixture also depends on the temperature, pressure and composition
4) 
b)  is the spectral absorption coefficient of the medium whose unit is m-1
is the spectral absorption coefficient of the medium whose unit is m-1
c) Separating the variables and integrating from x = 0 to x = L gives

Where we have assumed the absorptivity of the medium to be independent of x. Note that radiation intensity decays exponentially in accordance with Beer’s law.
d) The spectral transmissivity of a medium can be defined as the ratio of the intensity of radiation leaving the medium to that entering the medium

e) Radiation passing through a non scattering (and thus nonreflecting) medium is either absorbed or transmitted. Hence  the spectral absorptivity of a medium of thickness L is
the spectral absorptivity of a medium of thickness L is

f) From Kirchoff’s law, the spectral emissivity of the medium is