Introduction
Invertebrates constitute more than ninety-five per cent of the existing biodiversity of the world. They present an extreme range of variation in terms of their body architecture, adaptation, food preference, habitat preference, behaviour and physiological response against environmental stressors. Evolutionarily, they are an ancient group of animals which have survived an extreme range of environmental adversities and perturbations from the time of their origin. Invertebrates occupied diverse kinds of habitats including terrestrial, freshwater, marine and estuarine ecosystems. Aquatic invertebrates are usually distributed in the multiple spatial compartments of the water bodies from the surface to the bottom region called the benthic stratum. Aquatic invertebrates are also found in the conspicuous ecological areas of the mud–water interface and subsoil region at the floor of aquifers. Freshwater ponds of India serve as habitat for diverse aquatic organisms including members of Porifera, Mollusca and Arthropoda. Majority of the ecotoxicological research was carried out on Eunapius carteri (Porifera: Demospongiae: Spongillidae); Bellamya bengalensis (Mollusca: Gastropoda: Prosobranchia); Pila globosa (Mollusca: Gastropoda: Ampullariidae) and Lamellidens marginalis(Mollusca: Bivalvia: Eulamellibranchiata). Aquatic invertebrates bear ecological, economical, nutritional and biotechnological significance and demand a special scientific attention. Many of them are the dietary source of nutrition for human and other organisms.
Their importance as potential source of bioactive substances and pharmacologically active compounds cannot be denied. Current scientific reports indicate that the rapid and unrestricted contamination of freshwater and marine ecosystem by diverse pollutants poses serious ecotoxicological threat for the existence of invertebrates in their natural habitats. Various anthropogenic activities like habitat destruction and habitat contamination have been identified as the causative factors of dwindling of invertebrate species on earth. Continuous addition of various toxic chemical compounds into the global environment is a major environmental challenge encountered by human and other organisms. Many of these environmental pollutants are of either industrial or agricultural origin and need a thorough toxicological screening in invertebrate models. The overall evolutionary success and survival efficacy of invertebrates depended on several factors including the development of a highly advanced immunological system. Immunology deals with the typical strategies of biological defence against environmental toxins and pathogens. Components of immune system and their coherent functional attributes enabled the invertebrates to overcome the toxin-induced chemical stresses of the primitive and modern global environment (Figure 1). Many of the environmental pollutants of the current hydrosphere are less researched, with limited toxicological information. Invertebrates over a period of time are assumed to evolve novel and unique modes of immunological reactivities to defend against the toxic insults of environmental pollutants at the cellular and molecular levels. Aquatic invertebrates are relatively a neglected group of organisms with reference to their immunotoxicological status in a biounsafe environment. The principal immunotoxicological responsiveness of invertebrates centre around the reactivities of circulating immunocytes and selected effector organs like gill, digestive gland, intestine, labial palp, etc.
Immunotoxins and their impact on aquatic invertebrates
Immunotoxins are chemical compounds that can modulate the immune-related parameters and can adversely affect the biological and physiological functioning of the immune system of organisms including human. The freshwater ecosystem of India is being contaminated by diverse groups of xenobiotics of known and unknown chemistry. Ray et al. (2011) reported mineral acids, alkalis, detergents, metalloids and pesticides as major environmental contaminants of the Indian freshwater ecosystem [1]. Scientists reported [2] the toxic effects of pyrethroids, cypermethrin and fenvalerate in aquatic molluscan invertebrates and claimed them as immunotoxins for their adverse effect on the immune parameters of host animal. Toxicity of these pyrethroid pesticides on non-target aquatic invertebrates appears to be detrimental and can seriously affect their survival efficacy and reproductive success. Arsenic, another metalloid and a potent immunotoxin, bears toxic effects on aquatic invertebrates and can modulate the histopathology and immune parameters of freshwater mollusc [3] and estuarine mud crab [4]. Biopesticide azadirachtin has been recently identified as an immunotoxin due to its potential to affect the immunofunctioning of mussels. Moreover, during monsoon and flood, pesticide-laden agricultural runoff often contaminates the freshwater ecosystem and poses serious threat to its inhabitants. Washing soda, chemically known as anhydrous sodium carbonate, is an important aquatoxin that can alter the selected physiological parameters of diverse groups of invertebrates. Mukherjee et al. (2015b) reported the toxicity of washing soda in a freshwater sponge of India with reference to its phagocytic and cytotoxic status [5]. Altered functioning of the immune system may lead to opportunistic invasion of environmental pathogens and parasites into the body of host and increase the vulnerability of these biofilter species in polluted environment. The nature and magnitude of immunotoxicity depend on multiple parameters including the concentration of toxin, type and span of exposure and route of entry to the host. Immunotoxins are difficult to identify as they can cause a wide magnitude of adversity on the immune status of organisms. Immunotoxicology deals with the assessment of toxicological response in an organism by estimating the responsiveness and reactivity of its immunological parameters. Immunotoxicology of invertebrates, in recent times, has been gaining a special scientific attention for its efficacy in monitoring the health of environment – both aquatic and terrestrial. Accuracy and precision of selected toxicological responses of aquatic invertebrates enabled a few species to function as suitable biomonitoring agents of water pollution [6]. Several effective immunomarkers of aquatic pollution have recently been established in model invertebrates [7].

External physicochemical barriers of invertebrates as first line of immune defence against pollutants
External physicochemical barriers of the invertebrates act as the first line of defence against the invasion of parasite, pathogen and toxin in a polluted aquatic environment. External physicochemical barriers of the invertebrates include shell, tunic, test, cuticle, carapace, pinacoderm and others. In mollusc, hard calcareous shell or valves provide the primary protection against entry of pathogens and toxins. However, in shell-less molluscs, thick external cuticular sheet known as mantle or pallium are assumed to play a significant physiological role against toxin and pathogen entry. Soft body wall of molluscs consists of cuticle, epidermis and muscles, which are thought to take an active part in the innate immune defence against environmental pathogens and toxins. In a polluted environment, where environmental pathogens and toxins are present in sufficient quantity, the two valves of the mussel are kept closed to minimize the entry of undesired agents. In addition to external shell, mucus secreted from the internal viscera provides another line of defence against the invasion of pathogens and toxins.
Mucus acts as a protective barrier preventing the direct contact of toxins to epithelia. Secretion of mucus is reported to be an important detoxification and evasive mechanism of invertebrates. Pathogens are trapped within the mucus secreted by the organism leading to elimination. Calcareous shell made up of calcium carbonate and mucus is considered as external physiochemical barrier and plays an important role in the immune defence of aquatic invertebrates. Carapace, the external physiochemical barrier of crab provides the first line of defence in crustaceans. Corrosive toxins including mineral acids, alkalis, pesticides and detergents appear to be the potential threats of the invertebrate. Contamination of water bodies by these pollutants often results in breaches of the physicochemical barriers of external body surface, which leads to facilitation of invasion of toxic microorganisms and parasites into the viscera of target invertebrates. Components of innate immune response comprise physicochemical barrier against external pathogen and parasite entry. Cuticle covered with waxy material serves as a mechanical barrier of crab against parasitic infection. This chitinous exoskeleton or cuticle helps in the process of wound healing by preventing the fatal loss of haemolymph from body, maintenance of tissue architecture and prevention of opportunistic invasion of pathogen. Whenever this cuticle is damaged by injury or infection, the wound is rapidly sealed by clotting of immunocytes, preventing blood loss and pollutant entry. Once the clot is formed, wound is darkened and accumulation of melanin occurs. Melanin is reported to be involved in sealing of wound and synthesis of new cuticle. On the other hand, pinacoderm made up of flattened pinacocytes forms a continuous layer on the external surface of freshwater sponges and acts as a first line of defence against foreign invaders.
An experiment was performed to investigate the possible role of mucus as a primary barrier in removal of external particles. In this context, the shell of freshwater gastropod, P. globosa was drilled to create a micropore and a solution of cultured yeast (Saccharomyces cerevisiae) suspended in sterile phosphate buffered saline was injected into the body through a syringe (Figure 2). The mollusc was under scientific surveillance and the activity of the mollusc was photodocumented. After two hours of injection, hypersecretion of mucus was recorded by the experimental P. globosa within a time span of approximately thirty minutes. The expelled mucus contained more than ninety-five per cent of yeast particles, indicating the efficacy of mucus to act as external physicochemical barrier

Xenobiotic-induced shift in haemocyte density and morphological damages of blood cells
Haemocytes are the immunocompetent cells which are functionally responsive to various xenobiotics present in the aquatic environment. Homeostasis of total haemocyte density of aquatic invertebrates within the permissible physiological limit may be considered as an important immunological parameter [8] of cell-mediated immune response of molluscs [2]. Chakraborty et al. (2008) reported suppression in the total count of haemocytes of the freshwater edible mollusc, L. marginalis under the sublethal exposure of sodium arsenite, an aquatic pollutant [9]. Mukherjee et al. (2006) reported modulation in the total cell density of the same specimen under the sublethal concentrations of azadirachtin, a neem-based pesticide, a common contaminant of pond water [10]. According to them, toxin-induced alteration in the total cell dynamics may lead to a gradual decline of this species in its natural habitat. The total count of haemocyte of Villorita cyprinoides was found to decrease under the exposure of copper [11]. Ray et al. (2013b) reported dynamics of the total haemocyte density of B. bengalensis and L. marginalisexposed to environmentally realistic sublethal concentrations of cypermethrin and fenvalerate, respectively [2]. Authors reported a significant increase in total haemocyte count following exposure to experimental concentrations of cypermethrin and fenvalerate to B. bengalensis and L. marginalis, respectively. Russo and Madec (2007) reported effect of pollutant fomesafen pesticide on the haemocyte density in the snail, Lymnaea stagnalis [12]. Authors reported the increase in total number of circulating haemocytes under the exposure of fomesafen as “facilitated cell turnover.” The differential cell count, on the other hand, is also considered as an important immunotoxicological marker of environmental pollution [13]. Moreover, heterogenous populations of invertebrate blood cells have been reported to perform diverse physiological activities including nonself recognition, encapsulation and generation of cytotoxic molecules. Chakraborty et al. (2008) reported that haemocyte density can be considered as a potential biomarker of toxicity of sodium arsenite, a vital pollutant of the Sunderbans estuary of India [9]. Their experimental findings revealed the variation in the relative densities of granulocytes, agranulocytes, blast-like cells, hyalinocytes and asterocytes under the exposure of sodium arsenite. Ray et al. (2013b) reported a non-linear fluctuation in the differential cell density of B. bengalensis and L. marginalis following sublethal exposure of cypermethrin and fenvalerate, respectively [2]. According to them, aquatic pesticide-induced alteration in the differential haemocyte density was indicative to stress-induced loss of cell balance, which might alter the physiological homeostasis of these species distributed in pesticide-contaminated habitat. Qubella et al. (1993) proposed that fluctuation in the differential haemocyte density may be a result of reversible migration of haemocytes from tissues to haemolymph or vice versa [14].
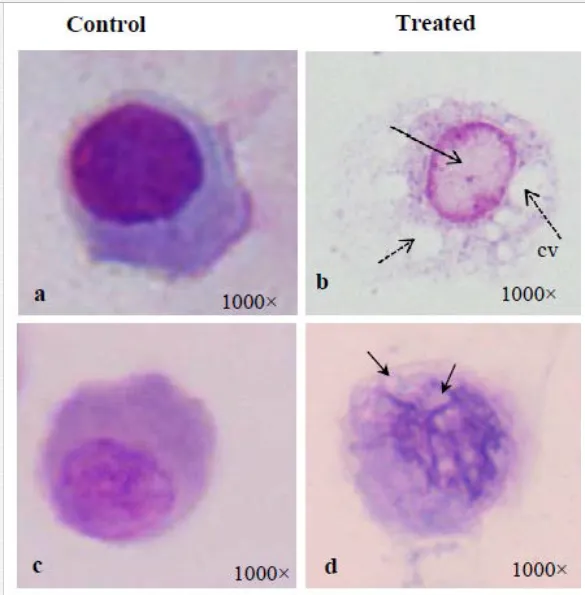
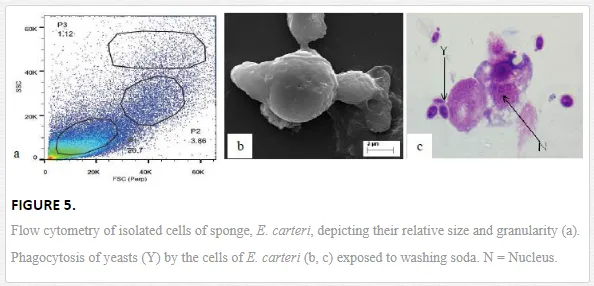
Mukherjee et al. (2008) reported an increase in relative percentage of pro-haemocytes subpopulation in aquatic mollusc L. marginalis under the sublethal exposure of azadirachtin [15], a contaminant of water bodies. According to them, pesticide-induced depletion in the densities of granulocytes, agranulocytes, hyalinocytes and asterocytes was indicative to a possible impairment of haemocytic function of mussels distributed in polluted environment. Ray et al. (2013b) flow cytometrically identified (Figure 5a) five discrete subpopulations of haemocytes, that is, blast-like cells, round hyalinocytes, spindle hyalinocytes, round granulocytes, and granular asterocytes in the freshwater molluscs, B. bengalensis and L. marginalis [2]. Experimental exposure of 0.5 ppm of cypermethrin for 96 hours resulted in significant increase in the per cent populations of blast-like cells and round hyalinocytes and significant decrease in the subpopulation of granular asterocyte in B. bengalensis as compared to control. Exposure of 3 ppm of fenvalerate for 96 hours resulted in significant increase in the relative percentage of round granulocyte and significant decrease in the subpopulation of round hyalinocyte in L. marginalis as compared to control. Das et al. (2012) reported alteration of relative densities of haemocyte subpopulations of L. marginalis under sublethal exposures of cypermethrin [16]. Exposure to sublethal concentrations of cypermethrin yielded decrease in densities of agranulocytes and asterocytes and increase in densities of blast-like cells and granulocytes. Pyrethroids-induced alterations in the differential cell density of freshwater molluscs may lead to impairment of blood cell homeostasis in the experimental species distributed in polluted environment. Morphological alterations of haemocytes under the exposure of environmental toxins play an important role in cell-mediated immune response of molluscs (Figure 3). Ray et al. (2013b) identified cytoplasmic hypervacuolation, rounding up of cell, alteration in cell shape, hypergranulation, increased cytoplasmic spreading, membrane disintegration and membrane blebbing as principal aberrations in the haemocytes of B. bengalensis and L. marginalis under the exposure of pollutant like pyrethroid [2]. Cypermethrin- and fenvalerate-induced morphological damages in the circulating haemocytes of freshwater edible molluscs may lead to possible impairment of the functioning of cells and their immunological reactivity. Chakraborty and Ray (2009) reported dose- and time-dependent increase in the frequency of binucleated and micronucleated haemocytes and gill cells in the freshwater bivalve, L. marginalis, under the exposure of sodium arsenite [6]. Authors claimed arsenic-induced nuclear anomalies may be used as a biomarker of arsenic toxicity in freshwater ecosystems.

Nonself surface adhesion and aggregation response of haemocytes
Nonself surface adhesion is considered as an important immunological mechanism which is fundamentally related to self–nonself discrimination. Cell adhesion and interaction between cell and the substratum play a pivotal role in the development, maintenance and immune recognition in multicellular animals. Mukherjee et al. (2007) studied the glass surface adhesion efficacy of haemocytes of L. marginalis under the sublethal concentrations of azadirachtin [17]. Authors reported a decrease in activity of surface adhesion property of haemocyte under prolonged exposure of pesticide. According to them, the data were indicative to impairment of immunological response of L. marginalis in its natural habitat leading to a possible dwindling of this biofilter species from aquatic ecosystem. Ghosh et al. (2008) studied the kinetics of nonself surface adhesion in the haemocytes of mud whelk, Telescopium telescopium exposed to diesel in the Sunderbans biosphere reserve [18]. Vehicle diesel is reported [19] to be a serious pollutant of Indian estuary. Authors reported diesel-induced inhibition in the nonself surface recognition efficacy and a shift in the kinetics of adhesion. Saha et al. (2008a) reported modulation in the nonself surface adhesion efficacy of haemocytes of edible mud crab, Scylla serrata, under the sublethal exposures of sodium arsenite [20]. Ray et al. (2012) reported arsenic-induced glass surface adhesion of haemocytes of juvenile mud crab [21]. According to them, arsenic-induced alteration in the reactivity of haemocyte may render the juvenile mud crab to become immunologically impaired in the parasite- and pathogen-contaminated natural habitat.
In the dynamic freshwater ecosystem, the inhabitants often compete for niche for their better survival and propagation. Overlapping in the niche leads to a state of acute predation and fighting among animals. As a result, the animals may encounter acute competition and subsequent physical damage and loss of body fluid. Cellular aggregation is a functional attribute offered by the haemocytes of invertebrates [22] to prevent the accidental blood loss by formation of biological plug at the site of wound and resist the entry of pathogenic microorganism. Selected pollutants like sodium arsenite, washing soda, pyrethroid, azadirachtin are reported to affect the aggregation response of many aquatic invertebrates (Figure 4). Hence, cell–cell aggregation is considered as an immunological response for host defence. Aggregation of haemocytes around invaded microorganisms is termed as “encapsulation response” and is considered as an important immunological response [23]. When successful encapsulation occurs, a host animal can restrict the proliferative and invasive property of a pathogen. Encapsulation reaction is mediated by specific population of immunoactive blood cells and is effective in cell-mediated immunity of invertebrates. Mukherjee et al. (2011) reported anticoagulant and carbohydrate-induced interference of cellular aggregation of mussel, L. marginalis, under experimental exposure of azadirachtin [24]. Persistent exposure of azadirachtin inhibited the cellular aggregation response in freshwater mussel. Workers apprehend such scenario in the natural environment may lead to a decline in the population of freshwater mussel and loss of freshwater biodiversity of India. Furthermore, a drastic increase in the occurrence of free cells was recorded against ethylene diamine tetraacetic acid and manure treatment, which was suggestive to possible role of these chemical agents as inhibitor of cellular aggregation. Ray et al. (2012) reported sodium-arsenite-induced inhibition in aggregation of haemocytes of juvenile mud crab, S. serrata [21]. According to them, arsenic-induced alteration of immune status may impart a state of vulnerability in juvenile crab inhabiting the arsenic-polluted environment.

Phagocytic response in the face of environmental stressors
Phagocytosis, in general, is considered a classical innate immune response reported in the majority of the invertebrate Phyla. It is an established immunological response and is considered as a biomarker of aquatic pollution [13]. Phagocytic response enables invertebrates to combat against invading pathogens and pollutants of known and unknown chemistry. Haemocyte-mediated phagocytosis of nonself particles provides natural immunity in the bivalves [25]. Chakraborty et al. (2009) reported the inhibitory effect of sodium arsenite on the phagocytic response of L. marginalis under the challenge of yeast at various sublethal concentrations [7]. Inhibition in phagocytic response was also recorded under similar laboratory condition for the haemocytes of arsenic-treated L. marginalis when challenged with human red blood corpuscles [26]. According to them, impairment in the phagocytic potential of the haemocytes of arsenic-treated mussels may lead to compromisation of the immune status of the animals distributed in the contaminated habitat. Mukherjee et al. (2011) reported azadirachtin-induced suppression in the phagocytic potential of haemocytes of L. marginalis under the experimental challenge of charcoal particulate [27]. Azadirachtin-induced inhibition in the phagocytic response indicated a state of immune suppression of mussels. Ray et al. (2012) screened the immunotoxicological reactivity of haemocytes of juvenile mud crab, S. serrata of the Sunderbans biosphere reserve and reported sodium-arsenite-induced inhibition in the phagocytic potential under the challenge of yeast [21]. Arsenic-induced altered reactivity of haemocytes may affect the propagation and survival of mud crab population by increasing its vulnerability to higher rate of disease and parasite attack. Sponges, on the other hand, are non-selective filter feeders which depend on phagocytosis for the purposes of feeding and digestion (Figure 5b, c). Phagocytosis, in sponge, plays a dual physiological attribute in the form of food procurement and innate immune response [28]. Mukherjee et al. (2015b) reported washing-soda-induced inhibition in the phagocytic potential of cells of freshwater sponge, E. carteri, under the challenge of yeast [5]. According to them, decrease in phagocytic potential is suggestive to a possible impairment of both food capture efficiency and innate immune status, leading to suppression of immunological status of E. carteri distributed in detergent-contaminated natural habitat.
Cytotoxicity of blood cells as an effective immune strategy
Cytotoxic molecules generated by the immunocompetent cells of invertebrates are reported to play an important role in the destruction and deactivation of foreign engulfed pathogens [29]. Authors reported superoxide anion, nitric oxide and phenoloxidase as established cytotoxic molecules of freshwater molluscs affected by various environmental pollutants. Contamination of natural habitat by toxic metals, metalloids, pesticides and washing soda results in a significant alteration in the cytotoxic status of invertebrates. Cytotoxic molecules are considered as an effective component of innate immune defence of invertebrates distributed in polluted environment. Nappi and Ottaviani (2000) reported nitric oxide and superoxide anions as potential “killing agents” of invertebrates [30]. Generation of reactive oxygen intermediate and reactive nitrogen intermediate by the phagocytic cells are mediated by NADPH oxidase and nitric oxide synthase (NOS), respectively. According to them, generation of superoxide anions can be correlated with increased respiratory burst activity in phagocytic cells. Mukherjee et al. (2012) reported a dose-dependent increase in the generation of superoxide anion in the cells of freshwater edible bivalve, L. marginalis under the exposure of azadirachtin, a neem-based pesticide [31]. According to them, this response of haemocytes may be considered as cellular stress under the sublethal and environmentally realistic concentrations of azadirachtin. Nitric oxide is considered as a signalling molecule generated in the biological system by the immunocytes of invertebrates as a potent cytotoxic agent for killing of invading microorganisms. It is generated during the conversion of L-arginine to L-citrulline by nitric oxide synthase. Saha et al. (2008b) reported sodium-arsenite-induced increase in the generation of the intracellular nitric oxide in estuarine mud crab, S. serrata [32]. According to them, dose-dependent increase in the generation of nitric oxide may be considered as a biomarker of arsenic pollution in the Sunderbans delta of India. Chakraborty et al. (2009) reported inhibition in generation of nitric oxide in L. marginalis under prolonged exposure of arsenic [7]. Authors claimed this studied parameter as possible biomarker of arsenic toxicity in aquatic environment. Phenoloxidase, on the other hand, is considered as another cytotoxic molecule and is involved in the process of nonself recognition, phagocytosis and melanisation. The production of toxic quinoid derivative by phenoloxidase is an early step of biosynthesis of melanin for host defence. Chakraborty et al. (2010a) reported depletion in generation of cytotoxic molecule like nitric oxide and activity of phenoloxidase in the gill of freshwater bivalve, L. marginalis, under the sublethal exposures of sodium arsenite [33]. According to them, sodium-arsenite-induced alteration in the cytotoxic status in gill indicated a state of immunocompromisation in the animal. Chakraborty et al. (2013) reported inhibition in the generation of superoxide anions and nitric oxide and activity of phenoloxidase in the digestive gland of the same experimental bivalve under prolonged exposure of arsenic [34]. Generation of cytotoxic molecules like superoxide anions and nitric oxide and activity of phenoloxidase are important immunological responses offered by the cells of sponge under the exposure of pathogens and toxins [5]. Authors reported washing-soda-induced alteration in the generation of superoxide anion, nitric oxide and activity of phenoloxidase in the dissociated cells of freshwater sponge, E. carteri.Washing-soda-induced alteration in cytotoxic response of E. carteri may lead to an undesirable shift in the immune status of the animal distributed in detergent contaminated natural habitat.
Lysosomal membrane stability and activity of phosphatases
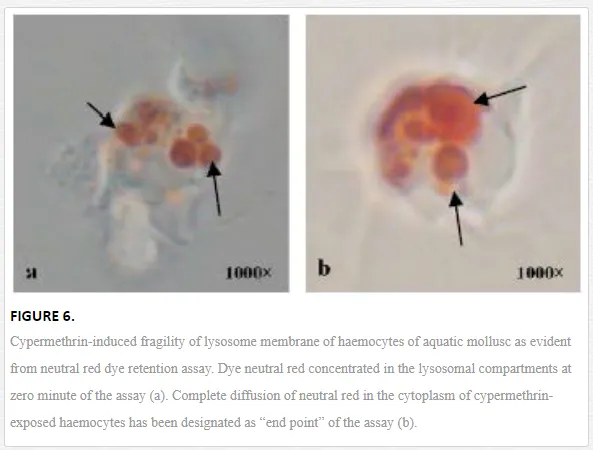
Lysosome is an important subcellular organelle involved in the process of degradation of foreign engulfed particulate. After phagocytosis, the phagocytic vacuole with engulfed foreign particles fuses with lysosome to form phagolysosome. As lysosome plays an important role in secretion of various digesting enzymes, maintenance of lysosomal membrane integrity has been gaining special scientific attention from the immunological point of view. Chakraborty and Ray (2009) reported impairment of lysosomal membrane integrity by neutral red retention assay in the haemocytes of L. marginalisexposed to sodium arsenite [6]. According to them, arsenic-induced fragility of lysosomal membrane may lead to leakage of lysosomal enzymes into the cytosol and subsequent destruction of the adjoining self cell or tissue. Ray et al. (2013b) reported lysosomal membrane integrity of haemocytes of B. bengalensis and L. marginalis under the experimental exposures of cypermethrin and fenvalerate, respectively [2]. Authors reported pyrethroid-induced lysosomal membrane fragility of haemocytes suggesting substantial damage and destabilization of lysosomal membrane (Figure 6). Screening of lysosomal membrane integrity of molluscan haemocytes by neutral red retention assay is considered as a possible biological marking of arsenic toxicity [6] and as an early warning tool of environmental pollution [35]. Phosphatases are principal lysosomal enzymes which are involved in pathogen destruction and are reported as markers of environmental stress [36]. Chakraborty et al. (2010a) reported suppression in the activity of hydrolytic enzymes, acid and alkaline phosphatases in the gill of freshwater edible bivalve, L. marginalis under the exposure of sodium arsenite [33]. According to them, inhibition in the activity of these enzymes might cripple the immune status and nutrient mobility in the gill of L. marginalis. Chakraborty et al. (2013) reported inhibition in the activity of phosphatases in the haemocyte and digestive tissue of edible bivalve under similar toxic insult by environmental arsenic [34]. Workers apprehend a state of immunocompromisation of the organisms under persistent exposure of arsenic. Saha et al. (2009) reported inhibition in the activity of phosphatases in the haemocytes of estuarine mud crab, S. serrata under the exposure of arsenic [37]. According to them, this situation in the natural environment might result in impairment of immunological activity and opportunistic invasion of parasite and pathogen into the body of the organism
Pollutant-induced apoptosis and necrosis of invertebrate immunocytes
Apoptosis, the programmed cell death, is functionally involved in developmental and immunological processes of organisms. Moreover, apoptosis helps in the process of removal of damaged cells and thus is considered an important machinery of survival of host animal in polluted environment. It is involved in specific cell signaling mechanism, which is yet to be studied in invertebrates in detail. However, pesticides and other environmental xenobiotics are reported to affect the apoptotic and necrotic pathways of the immunoactive cells of invertebrates (Figure 7). Current scientific reports suggest apoptosis as an effective biomarker of aquatic pollution. Apoptosis presented changes in the morphological characteristics of cells including membrane blebbing, nuclear condensation, cytoplasmic shrinkage and membrane asymmetry. Kiss (2010) reported the translocation of phosphatidylserine from inner leaflet of the plasma membrane to the outer leaftet, which was considered a hallmark of apoptosis [38]. Ray et al. (2013b) reported the apoptotic and necrotic cell deaths of haemocytes of B. bengalensisand L. marginalis under the sublethal exposures of cypermethrin and fenvalerate, respectively, employing flow cytometry [2]. According to them, cypermethrin and fenvalerate treatment yielded decrease in the percentage of apoptotic and necrotic haemocyte morphotypes of B. bengalensis and L. marginalis. Pyrethroid-induced apoptosis of molluscan haemocytes is considered as impairment of immunological status of B. bengalensis and L. marginalis.
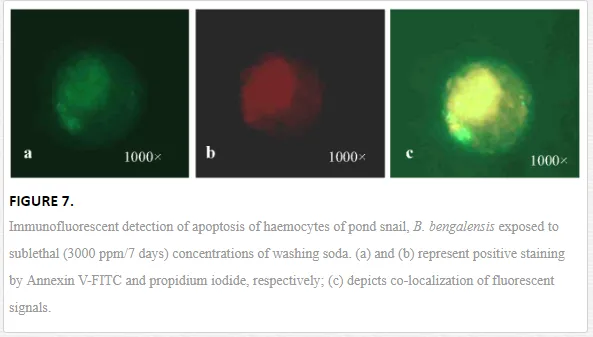
Toxin-induced damage of target organs and tissues
For filter feeding aquatic organisms, the gill, heart, digestive gland, mantle and antennae act as major target organs of common environmental xenobiotics (Figure 8). Mollusca bear a well-developed gill which serves as an organ of gaseous exchange, filter feeding and immunosurveillance. Molluscan gill is a thin membranous and vascularized organ that is in continuous contact with the environmental toxicants distributed in water. During the process of respiration and filter feeding, gill is exposed to various toxins of known and unknown chemistry. Exposure of gill to aquatic sodium arsenite, pyrethroid pesticide and washing soda of pond water yields a structural damage of lamellae. Chakraborty et al. (2010a) reported hyperchromatic anaplastic cells in the gills of L. marginalis exposed to sublethal concentrations of arsenic [33]. This is often associated with tissue rupture and formation of dense fibrosis. The heart, in general, acts as an organ of body fluid pumping. Exposure of cypermethrin results in severe histopathological damage of heart of mollusc. Similar kind of morphological damage was recorded due to exposure of aquatic mollusc to washing soda, a common pollutant of pond water. Digestive gland of mollusc bears immense physiological significance. The organ acts as an important site of diverse metabolic activities and biochemical detoxification. Exposure of sodium arsenite yields histopathological damage of the digestive gland of L. marginalis [34]. Toxin-induced morphological alteration of digestive gland of mollusc includes hyperinfiltration of haemocytes, vacuolation and inflammatory lysis.

Discussion
Global environment in recent times is characterised by the presence of various xenobiotics of known, less known and unknown toxicity and chemistry. Information of immunological attributes of chemical compounds in aquatic invertebrates is limited in the current scientific literature. Limited but significant reports indicate a substantial impairment of the immunological status of invertebrates under the exposure of selected ecotoxins (Figure 9). Acute, subchronic and chronic exposure of common toxins like pesticides, arsenic and alkaline washing soda cause severe damage in the morphological and functional profiles of haemocytes, the chief immunoeffector cells of invertebrates and other organs and tissues. Sponges, in general, are devoid of well-developed organ system. A variety of specialized cells of sponges are functionally involved in various immunological activities. Exposure of immunotoxins like washing soda largely affected the density dynamics of sponge cells as well as the cytotoxic and phagocytic status in freshwater sponge. Invertebrates of freshwater ecosystem act as important economical resource for developed and developing countries. A thorough toxicological analysis of the functional performance of target cells and tissues of invertebrates needs to be carried out for the purpose of conservation and culture of this species in their natural environment