Metal nanoparticles (NPs) nowadays represent the key material in vast range of industrial applications ranging from rough industry to fine medical or biochemical utilization. Gold and silver NPs and their applications are currently of great interest. In recent years, rapid developments in nanotechnology have evolved to the point where inclusion of those entities into smart systems and related technologies has become quite easy. Nowadays, NPs and NPs-embedded materials cover broad spectrum of functional devices with promising properties, which are used in many fields including optoelectronics or catalysis [1]. They are employed in modern nanobiotechnology for the production of biosensors, visualization of cellular structures [2], and targeted transporting of drugs [3]. Colloidal nanoparticles are studied because they have unique physical and chemical properties that are different from “bulk” materials [4]. In all applications of nanotechnology, the size and shape of the nanoparticles play an important role [5]. Numerous studies [6] describe the unique properties of gold nanoparticles which can be used in applications such as fuel cells, environmental, and other chemical processes [7]. Various preparation techniques have been proposed to cover specific requirements of those man-made entities. Besides classical wet-based preparation techniques of which the pioneering one is that proposed by Turkevich [8], others are intensely studied which would open up new potential applications of NPs in the development of new technologies. NPs are entities ranging in size between 1 to 100 nanometres.
These materials behave as a whole unit in terms of transportation and properties that highly depend on their shape, size, and morphological substructure [9, 10]. Those materials are characterized by high surface area-to-volume ratio, high chemical reactivity and physical affinity, as well as other interesting physico-chemical properties (optical, electrical, and magnetic) [11–14]. In addition, different NPs show various properties; likewise, different synthesis techniques of the same type of particle tend to alter or introduce new properties to the material. Consequently, the applications of these materials are unlimited and significantly remarkable in human life and in industries. Each nanomaterial with its distinct properties independently finds its way into practical applications such as: drug delivery [15], waste treatment [16], lubricant and surfactant production [17], electrical and sensor devices [18, 19], as well as membrane fuel cells [20]. Noble metal NPs have been widely studied in the past few decades because of their applications in various areas including catalysis, gas and biological sensors, drug delivery, and antimicrobial agents. In specific, due to their biocompatibility and photo-optical distinctiveness, gold NPs (AuNPs) have proven to be very useful tools in several biomedical applications dealing with different aspects of detection, analysis, and treatment. Recent advances in the study of noble metal NPs have led to their utilization in a number of very important applications including antimicrobial coatings, biosensing, diagnostic imaging, and cancer diagnosis and therapy [21–24]. This chapter surveys the various synthetic methods of broad spectrum of metallic NPs as well as most recent experimental studies focusing on the use of gold, silver, palladium, platinum, and ruthenium NPs in catalysis, food industry, and environmental applications. Moreover, the potent in vitro and in vivo antimicrobial and cytogenotoxic effects of various gold and silver nanomaterials are underlined. Finally, recent advances in functionalization of various solid substrates with gold and silver NPs as effective antimicrobial coatings and promising cell-stimulating agents are summarized. Despite their use in remediating numerous medical and health-related conditions, the efficacy and safety of many gold, silver, palladium, and platinum NPs are still under some scrutiny.
Nanoparticle synthesis
One of the most common nanoparticle synthesis of noble metals are those developed by Brust–Schiffrin [25]. The technique is based on reduction of AuIII+ complex compound with NaBH4 stabilized by thiols. This technique enables preparation of highly stable particles with narrow distribution and possibility to control their sizes. Also, Ag particles have already been synthesized on the basis of reduction reactions [7,8]. Concurrently, due to specific requirements on newly synthesized NPs, there have arisen other numerous techniques based on both wet and dry processes. Nowadays, most of the wet-based preparation techniques exploit confined reaction area inside which metal ions are reduced to zerovalent metals forming nanoparticles. The smaller and uniform the reaction cavity is, the smaller the dimension and narrower the size distribution of the resulting NPs. Different types of polymer-based agents have been investigated for preparation of metal and non-metal particles [26–31]. Metal NPs, semiconductors, inorganic oxides, and quantum dots synthesis were also investigated, especially in the confined space of surfactants, inorganic templates, and polymeric stabilizing agents [32–35]. The structure of the metal particles can be significantly affected by the preparation technique [36–39]. Their size, shape, and geometry strongly depend on the applied technique. Therefore, it is very important to use a template that is modifiable in terms of functional groups and is adjustable with respect to its shape or size. Polymeric hydrogels are able to fulfil these parameters, and they can be also successfully prepared in various sizes with different functional groups [26, 40]. Butun and Sahiner [41] reported synthesis of a novel bulk hydrogel based on acrylamidoglycolic acid and its use as a template in the preparation of different metal NPs (Ag, Cu, Ni, Co). Nanoparticle synthesis is based on reduction of the captured metal ions inside the hydrogel. Hydrogels play a key role in tissue engineering because of the resemblance of the three-dimensional network structure to the extracellular matrix environment [42–44]. The bulk hydrogels consist mostly of macropores (>50 nm) and even superpores with the pore sizes of up to several microns. The porosity can be modified by the degree of functional groups hydrophilicity or by the cross-linker used during preparation. The tune capability of the pore structure also provides specific advantages for the absorption and desorption of various species [45].
Completely new possibilities for the preparation of metal NPs open up utilization of PVD techniques. The most common capturing media for direct metal sputtering represent ion liquids (ILs) and their significantly less-toxic alternative – vegetable oils. First metal deposition onto liquid substrate was conducted in 1996 by the mean of radiofrequency (RF) magnetron sputtering. Silver was deposited into pure silicon oil [46]. The process went through two stages: (i) creation of percolation structure and (ii) nucleation of first metal clusters on the oil surface. With ongoing deposition, individual clusters spread out over the liquid surface and mutually interconnected forming continuous silver coverage floating on the oil surface. Such Ag layer exhibited considerable roughness with typical morphology. Sputtering process was strongly dependent on deposition conditions; when the sputtering power was held below 30 W, no silver layer was formed. Most probably, the drop of the sputtering power resulted in penetration of silver atoms into the liquid volume and formation of silver NPs (AgNPs). Nevertheless, this finding was not confirmed by the authors since their intention was to prepare ultra-thin silver layers on the surface of liquid [47].
Vegetable oils represent perspective liquid substrates for sputtering of metals, since they are commonly available, cheap, biocompatible, and have the ability to stabilize metal NPs [48] (e.g., by the way of chemical reduction [49]). Thus, for the successful preparation of nanoparticles, no other toxic reducing agents are required, which is very desirable for further in vivo applications. Direct sputtering of Au into castor oil results in formation of biocompatible AuNPs.
Application of higher voltage led to creation of larger particles, while the prolongation of deposition time had no impact on nanoparticle size, which was proved by transmission electron microscopy (TEM) and small angle Y-ray scattering (SAXS) [48]. Wender et al. [50] published interesting work correlating the properties of specific oil used with the deposition conditions with regards to formation of thin layers on the surface or in the volume of the oil. Authors showed that formation of AgNPs depends crucially on the applied voltage and specific surface coordination ability of the oil used (castor, canola, and kapron oil). Both lower voltage and weak coordination ability led to formation of continuous coatings on the oil surface. On contrary, higher voltage and strong coordination ability enable preparation of NPs. Higher voltage means higher diffusivity of adsorbed particles (metal atoms) on the surface of liquid which enables (i) particle penetration into the volume of liquid and (ii) anchoring of atoms/clusters to active functional groups. AgNPs were easily formed in castor oil (which contains solely hydroxyl groups) in wide range of applied voltage. On contrary, when using canola oil (which is predominately formed by unsaturated aliphatic chains) and kapron oil, thin silver layer was deposited on the oil surface at lower voltages, while AgNPs were formed only at higher discharge voltage.
Much more aggressive media (from the toxicity point of view) for potential biomedical application of prepared NPs are already mentioned ILs. Those liquids can be defined as liquid electrolytes exclusively composed of ions. Currently, the most common ILs are pure substances or eutectic mixtures of organic–inorganic salts, which melts at temperatures below 100°C [51]. Among the most widespread ILs belong derivates of organic molecules, e.g., pyrrolidine, imidazole, and pyridine [52]. ILs’ attractiveness in the process of NPs preparation consists in unusual physical–chemical properties. In addition to their extreme polarity, they also exhibit very low value of interface tension. Considering this, relatively high speed of nucleation can be achieved during the deposition process, which results in formation of small NPs without undesirable perturbations (Ostwald ripening[1] – ) [53]. Moreover, ILs very easily change their molecular arrangement so that to adapt to nucleation centres of emerging NPs, which leads to their stabilization. This high degree of adaptability consists in the presence of both hydrophilic and hydrophobic segments together with strong polarization force of ILs, which enables their orientation both perpendicular and parallel to the particles [13].
A large number of authors regard the value of surface tension and viscosity of liquid as critical in the process of preparation of metal NPs in liquid media. However, as evidenced in the work [52], in particular in the case of ILs, other parameters such as composition and the coordinating ability of the used liquid also play a significant role. If in addition the NPs growth occurs in the liquid volume, increase in the NPs size with increasing volume (size) of ILs anionic part can be expected [54–57]. Furthermore, it appears that the difference in size of the synthesized particles cannot be correlated with macroscopic characteristics of ILs, such as surface tension and viscosity [54]. In this connection, a very interesting medium for preparation of NPs by metal sputtering into liquid is pure propane-1,2,3-triole (glycerol) [31]. This substance combines physical properties comparable to those of ILs (i.e., low vapor pressure,thermally manageable viscosity, high coordination ability – per one glycerol molecule falls three hydroxyl groups) with minimal toxicity to living tissues. Biocompatibility of glycerol allows subsequent application of prepared NPs in bioengineering [58]. Additionally, one can easily control the size of emerging particles by the temperature of the capturing media (glycerol). Recent advances in preparation ways of selected noble metal NPs are summarized in Table 1.Besides all other noble metals, silver in the form of AgNPs possesses strong antimicrobial specificity. The AgNPs use is of great importance, since several pathogenic bacteria have developed resistance to different types of antibiotics. That is why AgNPs have emerged up with varied medical applications reaching from silver-based dressings, silver-coated medicinal devices (nanogels, nanolotions), etc. The antibacterial effects of Ag salts have been noticed since antiquity [59], and Ag is currently used to control bacterial growth in a wide spectrum of applications, including dental work, catheters, and burn wounds [60, 61]. In fact, it is well known that Ag ions and Ag-based compounds are highly toxic to microorganisms, showing strong biocidal effects on as many as more than ten species of bacteria including E. coli [62, 63].

Particle size reduction is an efficient and dependable tool to improve their bactericidal action and cytocompatibility. Nanotechnology techniques are of great assistance in the process of overcoming the limitations of size of nanoparticles [80]. Nanomaterials can be modified with the aim of better efficiency to facilitate their applications in various fields, e.g., bioscience and medicine.
Gold, silver, platinum, and palladium nanoparticles physically deposited into glycerol
In this section, a brief overview on results published by our group in the field of noble metal nanoparticles synthesis and investigation of their antibacterial action is introduced. The antimicrobial effects of Au, Ag, Pt, and PdNPs were tested against representative microorganisms of public concern. When applying metal NPs into living tissues, fundamental attention must be paid to their synthesis process itself. For successive, non-invasive application of metal NPs into living microorganisms, our group has developed a novel, unconventional approach for the physical synthesis of gold, silver, platinum, and palladium NPs based on direct metal sputtering into the liquid media (glycerol) [21, 31]. Considering this, direct metal deposition into the glycerol seems to be a promising technique combining the advantages of non-toxic and environmentally friendly process completely omitting the usage of solvents or reduction agents compared to classical wet-based methods. Furthermore, the possibility of tailoring the NP size via controlling the temperature of capturing media as well as the investigation of the functions of NP size and metal type (gold and silver) on the antibacterial activity are demonstrated. The antibacterial properties of these NPs were tested against two common pollutants of Escherichia coli(E. coli, Gram-negative bacteria) and Staphylococcus epidermidis (S. epidermidis, Gram-positive bacteria) naturally occurring on the skin and mucous membranes of human and frequently involved in infections associated with a biofilm formation. Prepared NPs were characterized by transmission electron microscopy with or without high-resolution (HRTEM, TEM) and UV–Vis spectroscopy.
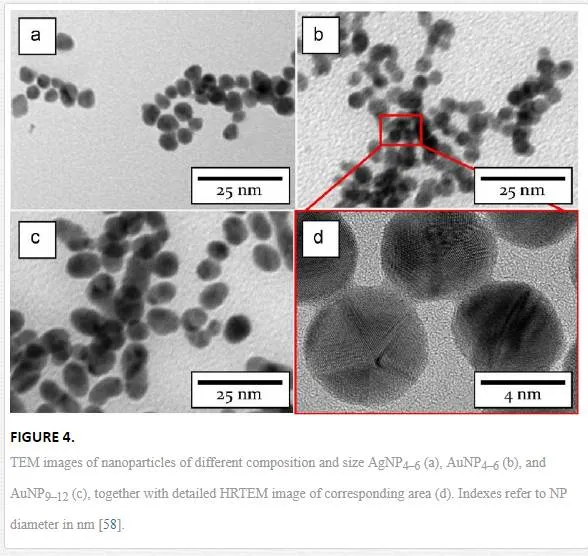
TEM images of Au and Ag NPs are shown in Figure 1 [31]. Both Au and Ag NPs possess spherical shape with average diameter of about 3.5 and 2.4 nm, respectively. It was shown that the NPs size distribution and uniformity remain untouched even in diluted aqueous solutions up to glycerol:water ratio of 1:20. It is a well-known fact that the presence of glycerol –OH group stabilizes aqueous solution of AgNPs [81]. But the stabilization role of glycerol –OH groups in AuNPs is not yet fully understood. Inset in Figure 1A introduces TEM image of AuNPs, which were stored 3 months at ambient conditions. The agglomeration of particles can be seen, which is in accordance with UV–Vis analysis. Contrary to that, this phenomenon was not found in case of AgNPs.

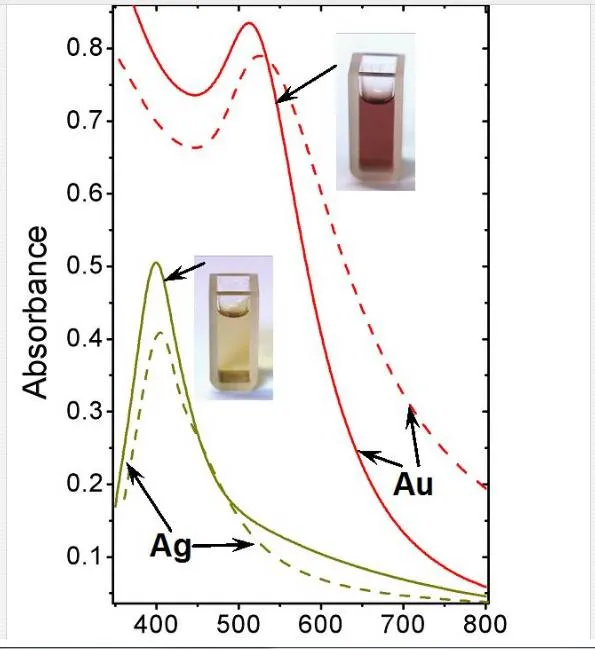
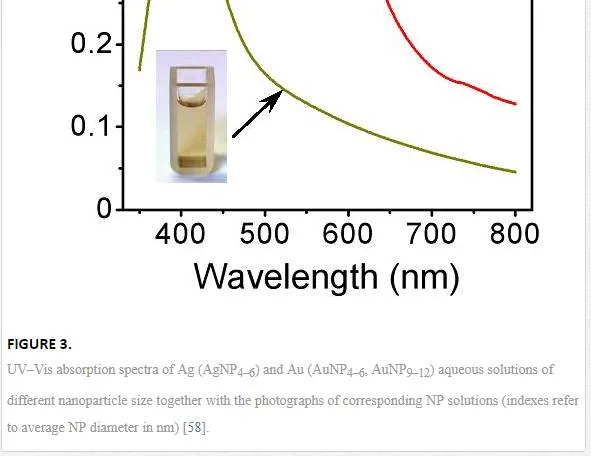
UV–Vis absorption spectra measured on Au and AgNPs solutions promptly after deposition (solid lines) and after 3 month storage at ambient conditions (dash lines) are introduced in Figure 2 and discussed in detail in [31]. UV–Vis is often used to estimate size, shape, and particle size distribution of colloidal solutions of metal nanoparticles as they show specific absorption band corresponding to localized surface plasmon resonance (LSPR) [82, 83]. Nevertheless, this method must be conducted under precise and reproducible conditions, since the position and intensity of LSPR absorption peak are affected by many factors, e.g., used solvents, type of stabilizers, and counter ions. The shape of the spectra exhibiting significant LSPR peaks corresponds to uniform nanoparticle colloidal solutions and also corresponds to the narrow size distribution [12, 84]. UV–Vis absorption spectrum of Au sample measured directly after deposition (solid line) shows diminishing LSPR peak with maximum at 512 nm. The shape and position of this peak suggest the particle sizes to be in interval from 2 to 10 nm [82], which corresponds with the sample’s type (aqueous solution). Nevertheless, the influence of the AuNPs stabilization by –OH groups on the peak shift is not yet fully known. Mild red shift in spectrum after 3 month storage indicates both agglomeration effect and less narrow distribution compared to original sample. Narrow and high-intensity peak occurring at 400 nm together with the pure yellow coloration of the colloid solution indicates the presence of zerovalent Ag. Oxidized AgNPs do not exhibit this peak in their UV–Vis spectrum [85]. Contrary to AuNPs, AgNPs solution is much more stable even after 3 months storage. The corresponding spectrum exhibits almost the same peak position with pretty narrow distribution.
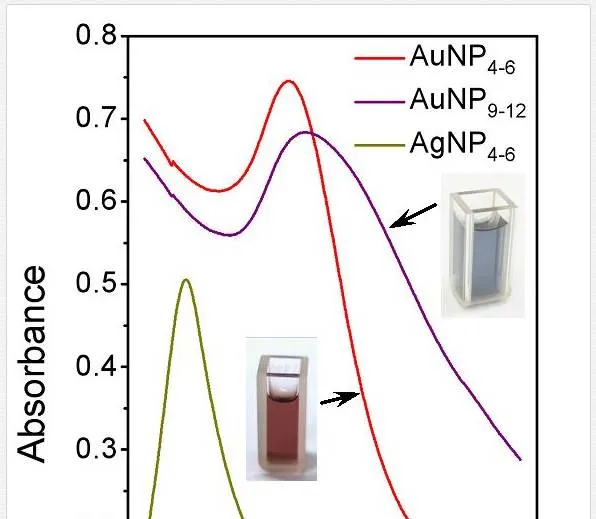
Targeted variation of metal nanoparticle size belongs to the great challenges of current science. Variation of capturing media temperature may provide a broad spectrum of nanoparticle diameters. The control of nanoparticle size through the capturing media temperature may be expressed upon a simple, generally known approach [86], which states that nanoparticle growth is governed by diffusion from the bulk of colloidal dispersion to the solution/particle interphase. When applied this approach, both AgNP4–6 and AuNP4–6 (indexes refer to average nanoparticle size) exhibit distinctive narrow peaks with maximum absorption at 400 and 520 nm, respectively (see Figure 3) [58]. These spectra are almost identical to those obtained in our former study on Ag and Au with average diameter of 3–5 nm, indicating stable solutions with minimal particle dispersion. Considerable broadening with pronounced red shift of absorption peek occurs at AuNP9–12. This phenomenon originates from both, increase in dimensions of individual Au particles and broader size distribution compared to AuNP4–6. Distinctive red coloration of AuNP4–6 turns into purple one, belonging to AuNP9–12, as the average size increases from ca 5 to 10 nm (see Figure 4).

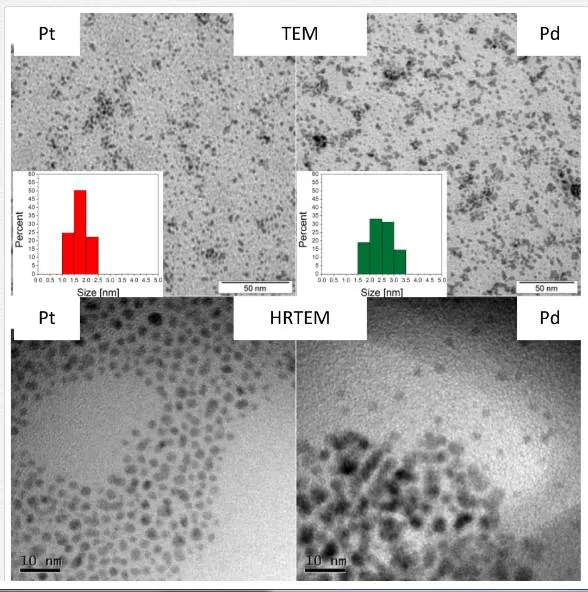
Generally, two major aspects determine whether the growth of NPs during metal sputtering into liquids will take a place rather than formation of thin film on the liquid surface [52]. Firstly, the kinetic energy of sputtered atoms or clusters must be sufficient to penetrate the surface of capturing media. Secondly, the first condition may be fulfilled only in the limited range of media viscosity; thus, the liquid media cannot be too viscous. When those conditions are met, the formation of NPs occurs. Figure 5 shows TEM and HRTEM images of diluted aqueous solutions of Pt and PdNPs. Image analysis of more than 500 particles from 10 different areas of TEM pictures proved that prepared PtNPs and PdNPs have average diameter of (1.7 ± 0.3) nm and (2.4 ± 0.4) nm, respectively. Apparent aggregation of particles is due to the preparation method of samples for TEM (HRTEM) analysis. Observed discrepancy in particle size is probably due to different sputtering yield of both metals (Pt ~ 1.27, Pd ~ 2.09) [87], which is in accordance with supposed growth mechanism causes considerably slower growth of Pt particles (lower concentration gradient).
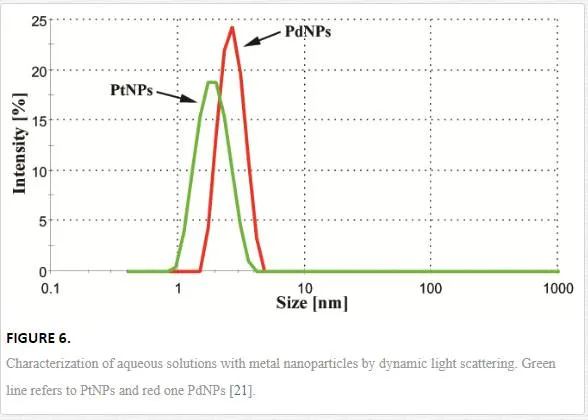
Additionally to TEM analysis, DLS measurement was accomplished to determine average size and size distribution of prepared NPs (see Figure 6, previous page). DLS measurements indicates that both particles are about 7% bigger compared to TEM-based analysis, which is in a good agreement with the published results [88], since DLS technique provides hydrodynamic diameter. Moreover, observed size discrepancy is inherently caused by intensity weighted mean particle diameter in case of DLS contrary to number weighted diameter obtained by TEM analysis [11]. More importantly, DLS proves that prepared NPs are not agglomerated, which is of crucial importance for evaluation of their bactericidal effects.

Formation and stability of metal NPs in aqueous colloidal solution were confirmed by UV–Vis spectroscopy. Figure 7 shows the UV–Vis spectra recorded from the Pd and PtNPs immediately after NPs preparation (solid lines) and after 3 months storage at laboratory conditions (dashed lines). It can be seen that apart from a distinct surface plasmon resonance (SPR) characteristic for other noble metal NPs (Au and Ag) [58], no distinctive SPR peaks occur in case of both Pd and PtNPs, which is in accordance with the earlier reports [89, 90]. Studied metal NPs exhibit increasing absorption toward shorter wavelengths [89, 91–93]. The course of dependence in case of both Pt and PdNPs indicates the presence of zerovalent metals in the solution [91]. Slight shift of the curves to lower absorbances at red region of visible spectrum (dashed lines) refers to mild agglomeration of individual particles in the solution after 3 months of storage. Bactericidal potency of various metal NPs has been previously reported [94–97]. It is known that their antimicrobial potency is governed by their size, composition, surface area, and charge [98, 99].
Antibacterial effects of noble metal nanoparticles
Antibacterial properties of Ag, Au, Pd, and PtNPs were examined using two bacterial strains, E. coliand S. epidermidis, frequently involved in infections associated with a biofilm formation. Demonstration of inhibition effect of AgNP4–6 on S. epidermidis is shown in Figure 8. The growth of E. coli and S. epidermidis was completely inhibited in the presence of AgNP4–6 after 24 h (for both 6 and 24 h incubated samples) when compared to the control samples (bacteria incubated in glycerol or physiological saline solution [PBS]), see Table 2 and Figure 8 [58].
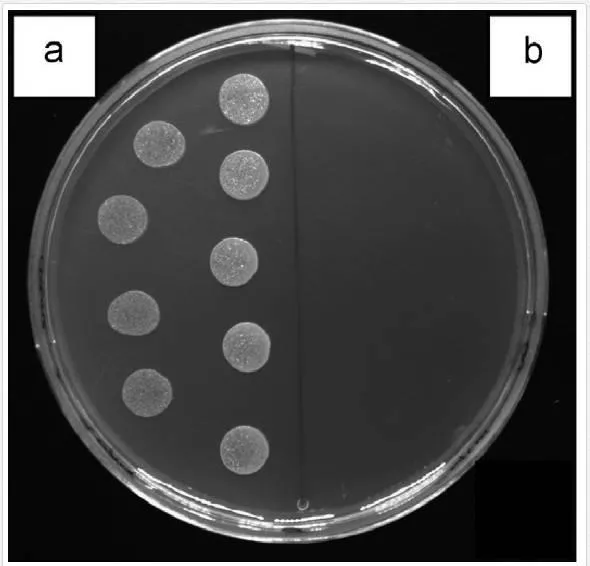
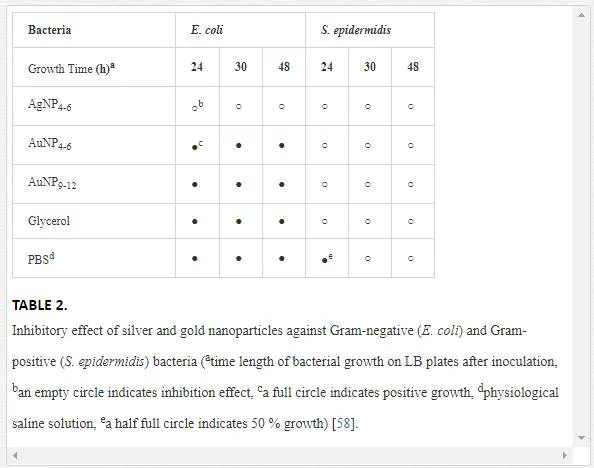
The growth inhibition of both bacterial strains was further maintained even after 30 and 48 h of growth, suggesting strong bactericidal activity of AgNP4–6. This is in contrary to generally accepted fact that Gram-negative bacteria are more susceptible to the inhibitory action of silver [100] caused probably by more facile penetration of silver through their thinner cell wall. On contrary, any growth inhibition was observed toward E. coli neither in the presence of AuNP4–6 nor in the presence of AuNP9–12 during the whole experiment. Surprisingly, AuNP4–6, but not AuNP9–12, were able to inhibit the growth of S. epidermidis and the effect was preserved for whole tested period (48 h), when compared to control samples. The antibacterial action of Ag+ ions has been broadly reported so far [101], also AgNPs repeatedly showed their potency against bacteria [102]. Thus, our results (growth inhibition of both bacterial strains by AgNP4–6) are in agreement with other groups results. Biocidal properties of AuNPs of similar size (5 nm) as prepared by our group (4–6 nm), were observed by Lima et al. [103]. Their AuNPs dispersed on zeolites were effective against Gram-negative E. coli and Salmonella typhi (90–95% growth inhibition). It has been reported that the antibacterial activity of AgNP is dependent on particle size and shape [104, 105]. Thus, it is very likely that also the size of AuNP plays significant role in the antimicrobial action.
Although there has been increased interest in studying bactericidal properties of noble metals over the past decades, only few have been reported on Pt and PdNPs. The inhibitory effect of one NPs concentration and four concentrations of bacteria was examined after 4 h in contact with NPs and 24 h of further post-incubation. From Figure 9, it is apparent that incubation of PdNPs with E. coli had pronounced effect on its growth up to 1 105 CFU per sample when compared to untreated control cells.

This remarkable antibacterial activity of Pd was diminished at higher bacterial concentrations ranging from 1 106 CFU per sample. Similar potency of PdNPs was observed against S. epidermidis (see Figure 9), even up to 1 106 CFU. Since PtNPs had very similar size as the first examined particles, the palladium ones, we expected comparable antimicrobial potential. Nevertheless, we detected only insignificant inhibition of bacterial growth induced by PtNPs at a concentration of 1 105 CFU of E. coliwhen compared to the control samples. Interestingly, we did not observe any growth inhibition of S. epidermidis in the presence of PtNPs. More importantly, we even recorded slight stimulation of their growth.
Grafting of noble metal nanoparticles to plasma-activated polymer carriers
Polymer surface can be considered as the phase boundary between the bulk polymer and the ambient environment. The application of polymeric materials depends significantly on the boundaries’ conditions. The most of polymeric materials, nevertheless, have chemically inert surface, mostly hydrophobic. Non-treated polymer surfaces exhibit very often poor performance in adhesion, coating, packaging, or colloid stabilization. [106–109].During the past few decades, the modifications of polymer surface were under intensive research for applications in various fields of industry. Different improved techniques including chemical or physical enhancements were applied. Physical processes possess the advantage of surface interaction with radiation of electromagnetic waves, and oxidation with gases. On the contrary, chemical modifications use wet-treatment, such as blending. This part of manuscript is focused on the review of recent advances in surface grafting of polymer substrates which can be performed by the combination of physical and chemical processes [107, 110]. The advantages of grafting procedures in comparison to other procedures may be summarized to several points, such as controllable introduction of graft chains with a high density, the precise localization of graft chains to the surface with almost no change in bulk’s surface properties. In contrast to physically coated polymer chains, covalent anchoring of graft chains onto a polymer surface avoids their delamination and assures their long-term chemical stability [111].
Minimally four strategies can be used for creation of functionalized polymer surfaces with gold nanostructures: (i) “grafting from procedure” is based on polymer chain growth from small initiators connected to AuNPs [112, 113]. This procedure leads to a very dense polymer brush construction. Other methods are based on radical polymerization (LRP) [114] and atom-transfer radical polymerization (SI-ATRP) [115, 116] (ii) “grafting to” enables one-pot synthesis of AuNPs stabilized by sulphur-containing polymers [10, 117], and generally produces a sparser coverage [118] (iii) physisorption using block copolymer micelles (nanoreactors), water-soluble polymers, or star block copolymers [119, 120] (iv) “post-modification of pre-formed AuNPs”. In this method, AuNPs are generated in the first stage through conventional methods such as Brust−Schiffrin synthesis, followed by the exchange or modification with polymers [121, 122].
This section is dedicated to description of two procedures of Au and AgNPs grafting on plasma-modified PET developed by our group. In the first procedure, Au and AgNPs were deposited on PET, beforehand grafted with biphenyl-4,4’-dithiol (BPD). In the second procedure, the Au and AgNPs (both denoted with*), beforehand grafted with BPD, were coated onto plasma-modified PET (see Figure 10).

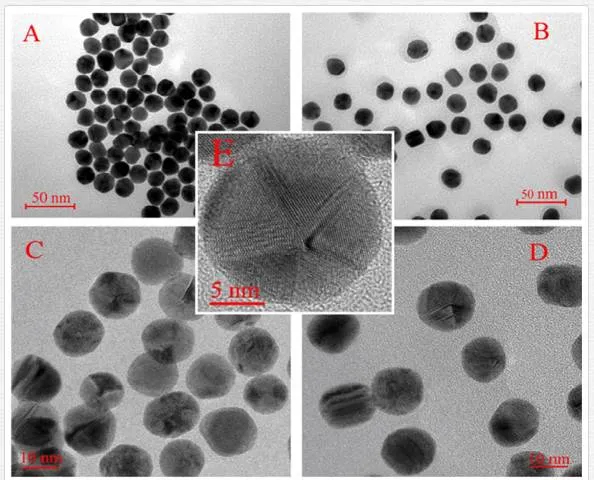
In Figure 11, one can see the images of AuNPs obtained by TEM and HRTEM. It is evident that the AuNPs size is the same before and after BPD coating and their average diameter is 14.7 nm. Grafting with BPD does not lead to AuNPs aggregation, thanks to the presence of hydrophilic groups on the NP surface. It is known that the gold grows in a square fcc crystal structure with dominant plane (111) [123]. At higher HRTEM resolution (Figure 11C,E), it is seen that the AuNPs are in the form of decahedral particles. As a result of deficiency, real AuNPs should contain defects or be intrinsically strained [124]. In Figure 10E, gold atoms arranged in (111) plane are clearly seen. From Figure 11D, it is seen that some of the form of BPD-coated AuNPs differs from that of uncoated AuNPs and they are not in the form of decahedral particles due to the decrease of the surface tension [125]. It is apparent that the behavior of pristine AgNPs (AgNP in Figure 11F) and AgNPs modified with BPD (AgNP* in Figure 11G) is significantly changed. AgNPs form uniform aggregates with nonspherical shape. On the contrary, AgNPs* form structures with spherical character which are well dispersed. Surface modification technique with BPD application does not result in AgNPs aggregation due to the presence of hydrophilic (-SH) and hydrophobic groups the nanoparticle‘s surface. The calculated diameters of AgNP and AgNP* were (55±10) and (45±10) nm, respectively [126].

Both (Au, Ag)NPs and (Au, Ag)NPs* in solution were characterized by UV–Vis spectroscopy (see Figure 12). The absorption spectrum of the AuNPs solution (see Figure 12A) shows a maximum around 521 nm, corresponding to the transverse plasmon oscillation band. The wavelength of the surface plasmon resonance (SPR) [127] corresponds well with the average diameter estimated from TEM images. SPR absorption wavelength is known to increase with nanoparticle size [124]. From Figure 12, it is obvious that the absorption maximum of AuNP* is shifted toward larger wave lengths. This finding is in accordance with the above mentioned fact that the BPD-coated AuNPs (AuNPs*) exhibit enlarged surface [128]. The UV–Vis absorption spectra of AgNPs and AgNPs* suspensions (Figure 12B) exhibit well-defined plasmon bands at about 455 and 413 nm, which is characteristic of nano-sized silver [31, 97, 129]. Observed wavelengths correspond well with average diameters of AgNPs estimated from TEM images (Figure 11F,G).


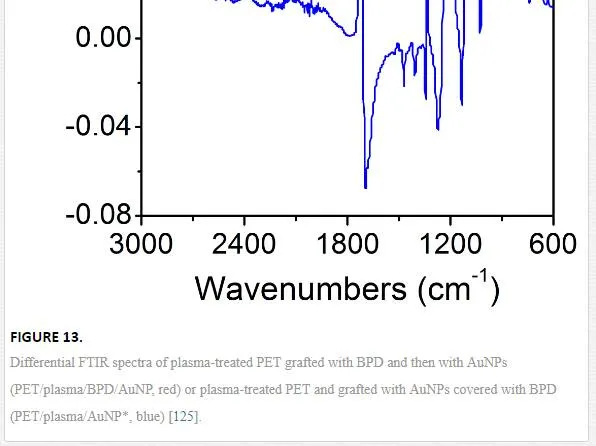
Differential FTIR spectroscopy was used for the characterization of pristine PET, plasma-treated PET, and PET grafted with BPD and AuNPs using both techniques described above. Typical difference FTIR spectra are shown in Figure 13. The band at 2634 cm–1 corresponds to residual dithiol group. The band at 715 cm–1 corresponds to the –S–Au group. So that, the FTIR measurement proves that the AuNPs, deposited using both procedures examined, are chemically bound to the plasma-activated PET surface. XPS analysis was used to monitor the change in the surface chemical composition after subsequent preparation steps. Element concentration of C, O, S, and Au on the surface of all samples (using both grafting techniques) is summarized in Table 3. After the plasma treatment, the PET surface is oxidized dramatically. The polymer oxidation after the plasma treatment and creation of oxygen-containing groups (C–O ~ 286.0 eV, O–C=O ~ 288.8 eV) at the polymer surface is well known [130]. Polymer chain is characterized by the C1s component centered at 284.7 eV due to C–C group. There is a change in the contribution of the shake-up satellite 290.9 eV due to the attachment of aromatic species (BPD) to the plasma-treated PET, see references [125, 131]. After grafting of plasma-treated PET with BPD, the oxygen concentration decreases dramatically. The attachment of BPD to the surface of PET (PET/BPD) was evidenced by the detection of the sulphur with concentration of 5.7 at. %. The concentration of bonded AuNP and AuNP* varies from 0.1 to 0.02 at. %, respectively. Atomic concentrations of C(1s), O(1s), S(2p), and Ag(3d) in pristine, plasma-modified PET and after grafting with BPD and silver nanoparticles are summarized in Table 3. After the next grafting procedure realized with AgNP and AgNP* particles, the concentration of sulphur decreased and silver was confirmed for the PET/plasma/BPD/AgNP samples, which indicates the presence of AgNPs on the surface of treated PET. In the case of PET/plasma/AgNP* samples, the silver concentration is presumably below the detection limit of XPS method. However, the evidence of successful AgNP* attachment was confirmed by the presence of sulphur on the treated surface.

It is known that the thiol groups bond to double bonds created on surface by plasma treatment by free radical chain mechanism [132]. Radicals created after plasma activation of PET are located mainly at heteroatoms (e.g., oxygen of ester or hydroxyl group). Therefore, these radicals are stable and do not stabilize by creation of double bonds on surface [133]. In our previous study [134], it has been proved the amount of free radicals created on PET surface modified by plasma is very low. That is the reason why more double bonds are not available on PET, and therefore the amount of bonded thiol groups is not much higher [125, 126].
Conclusions
This chapter attempts to provide a comprehensive insight into the problems of new techniques of preparation of noble metal NPs. It summarizes the basic information about the elementary characteristics and technology of preparation of those man-made materials. The introduction gives some basic information on the history of development in this area, especially in terms of dimensionality of metal nanostructures and their possible applications. General overview is supported by the specific research of the author team in this field. Presented findings support the hypothesis that Au, Ag, Pd, and PtNPs can be prepared in a simple and cost-effective manner and are suitable for formulation of new types of bactericidal materials. Presented approach is based on temperature control of capturing media during the sputtering deposition process into propane-1,2,3-triol, allowing targeted variation of NPs size. Bactericidal action of such synthesized NPs was examined against two common pollutants (E. coliand S. epidermidis). While AgNP4–6 exhibit strong potential to completely inhibit both bacterial strains after 24 h, AuNPs showed pronounced inhibition selectivity regarding the specific NP size. Regardless of AuNP size, any growth inhibition of E. coli was observed. Contrary to that, AuNP4–6 were able to inhibit the growth of S. epidermidis. We have also illustrated a significant difference in biological activities of Pt and PdNPs. More specifically, PdNPs exhibited considerable inhibitory potential against both E. coli and S. epidermidis, which was in contrast to ineffective PtNPs. Those findings indicate that palladium has high potential to combat both Gram-positive and Gram-negative bacterial strains. Understanding of nanoparticle–bacteria interactions is a key issue in their potential applications in clinics on everyday basis. Observed discrepancy in bactericidal action between Pt and Pd nanoparticles could be attributed to different size of individual particles. Finally, we have introduced procedures for PET surface coating with noble metal nanoparticles. The techniques were based on activation of PET surface with Ar plasma discharge and consequent application of dithiol as binding reagent between Au and Ag nanoparticles and plasma-treated PET. The introduction of gold and silver nanoparticle–thiol layer on the activated PET surface was confirmed with XPS analysis. Higher concentration of Au and AgNPs was achieved by deposition on PET grafted beforehand with dithiol.