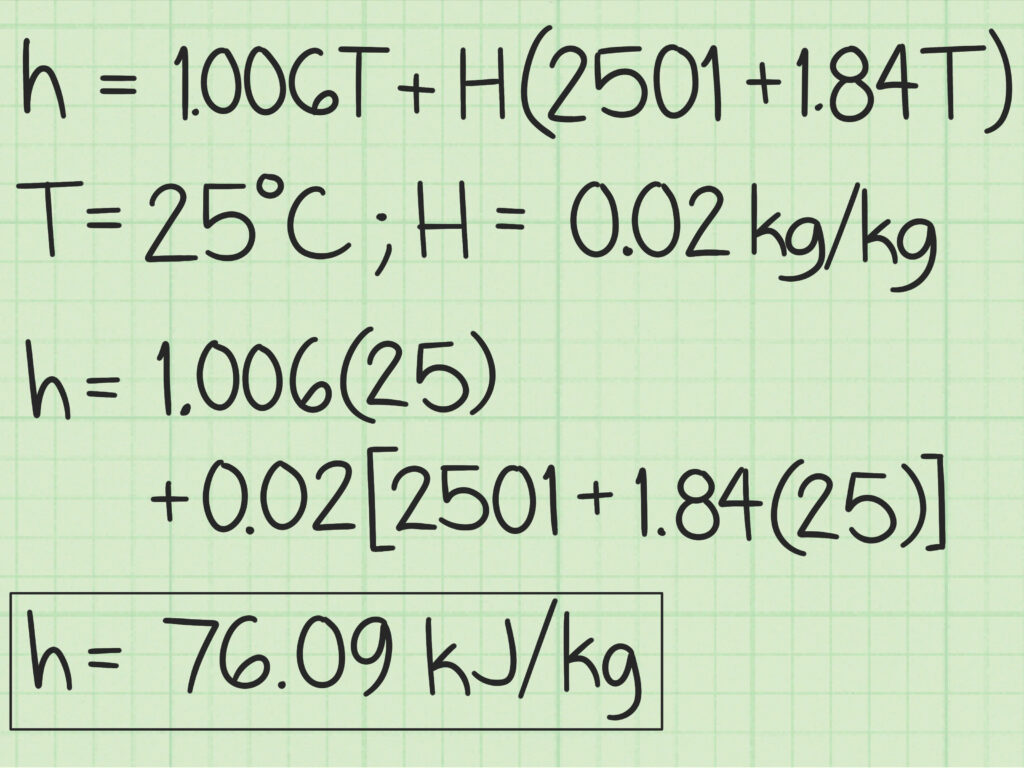In order to perform air conditioning calculations, it is essential first to estimate various properties of air. It is difficult to estimate the exact property values of moist air as it is a mixture of several permanent gases and water vapour. However, moist air upto 3 atm. pressure is found to obey perfect gas law with accuracy sufficient for engineering calculations. For higher accuracy Goff and Gratch tables can be used for estimating moist air properties. These tables are obtained using mixture models based on fundamental principles of statistical mechanics that take into account the real gas behaviour of dry air and water vapour. However, these tables are valid for a barometric pressure of 1 atm. only. Even though the calculation procedure is quite complex, using the mixture models it is possible to estimate moist air properties at other pressures also. However, since in most cases the pressures involved are low, one can apply the perfect gas model to estimate psychrometric properties.
Basic gas laws for moist air:
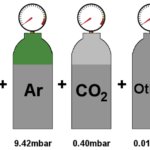
According to the Gibbs-Dalton law for a mixture of perfect gases, the total pressure exerted by the mixture is equal to the sum of partial pressures of the constituent gases. According to this law, for a homogeneous perfect gas mixture occupying a volume V and at temperature T, each constituent gas behaves as though the other gases are not present (i.e., there is no interaction between the gases). Each gas obeys perfect gas equation. Hence, the partial pressures exerted by each gas, p1,p2,p3 … and the total pressure pt are given by

where n1,n2,n3,… are the number of moles of gases 1,2,3,…
Applying this equation to moist air.