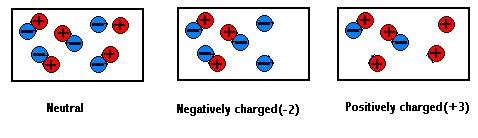
As discussed in a previous section of Lesson 1, atoms are the building blocks of matter. There are different types of atoms, known as elements. Atoms of each element are distinguished from each other by the number of protons that are present in their nucleus. An atom containing one proton is a hydrogen atom (H). An atom containing 6 protons is a carbon atom. And an atom containing 8 protons is an oxygen atom.
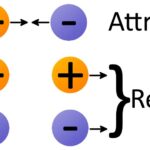
The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. A proton and an electron have an equal amount but an opposite type of charge. Thus, if an atom contains equal numbers of protons and electrons, the atom is described as beingelectrically neutral. On the other hand, if an atom has an unequal number of protons and electrons, then the atom is electrically charged (and in fact, is then referred to as an ion rather than an atom). Any particle, whether an atom, molecule or ion, that contains less electrons than protons is said to be positively charged. Conversely, any particle that contains more electrons than protons is said to be negatively charged.
| Charged versus Uncharged Particles | ||
| Positively Charged | Negatively Charged | Uncharged |
| Possesses more protons than electrons | Possesses more electrons than protons | Equal numbers of protons and electrons |
Charged Objects as an Imbalance of Protons and Electrons
In the previous section of Lesson 1, an atom was described as being a small and dense core of positively charged protons and neutral neutrons surrounded by shells of negatively charged electrons. The protons are tightly bound within the nucleus and not removable by ordinary measures. While the electrons are attracted to the protons of the nucleus, the addition of energy to an atom can persuade the electrons to leave an atom. Similarly, electrons within atoms of other materials can be persuaded to leave their own electron shells and become members of the electrons shells of other atoms of different materials. In short, electrons are migrants – constantly on the move and always ready to try out a new atomic environment.
 All objects are composed of these atoms. The electrons contained within the objects are prone to move or migrate to other objects. The process of an electron leaving one material object to reside (perhaps only temporarily) in another object is a common everyday occurrence. Even as you read the words of this web page, some electrons are likely moving through the monitor and adhering to your clothing (assuming that you are using this resource online) (and wearing clothes). If you were to walk across the carpeting towards the door of the room, electrons would likely be scuffed off the atoms of your shoes and moved onto the atoms of the carpet. And as clothes tumble in the dryer, it is highly likely that electrons on one piece of clothing will move from the atoms of the clothing onto the atoms of another piece of clothing. In general, for electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source, a motive, and a low-resistance pathway.
All objects are composed of these atoms. The electrons contained within the objects are prone to move or migrate to other objects. The process of an electron leaving one material object to reside (perhaps only temporarily) in another object is a common everyday occurrence. Even as you read the words of this web page, some electrons are likely moving through the monitor and adhering to your clothing (assuming that you are using this resource online) (and wearing clothes). If you were to walk across the carpeting towards the door of the room, electrons would likely be scuffed off the atoms of your shoes and moved onto the atoms of the carpet. And as clothes tumble in the dryer, it is highly likely that electrons on one piece of clothing will move from the atoms of the clothing onto the atoms of another piece of clothing. In general, for electrons to make a move from the atoms of one material to the atoms of another material, there must be an energy source, a motive, and a low-resistance pathway.
The cause and mechanisms by which this movement of electrons occurs will be the subject of Lesson 2. For now, it is sufficient to say that objects that are charged contain unequal numbers of protons and electrons. Charged objects have an imbalance of charge – either more negative electrons than positive protons or vice versa. And neutral objects have a balance of charge – equal numbers of protons and electrons. The principle stated earlier for atoms can be applied to objects. Objects with more electrons than protons are charged negatively; objects with fewer electrons than protons are charged positively.
In this discussion of electrically charged versus electrically neutral objects, the neutron has been neglected. Neutrons, being electrically neutral play no role in this unit. Their presence (or absence) will have no direct bearing upon whether an object is charged or uncharged. Their role in the atom is merely to provide stability to the nucleus, a subject not discussed in The Physics Classroom. When it comes to the drama of static electricity, electrons and protons become the main characters.
Charge as a Quantity
Like mass, the charge of an object is a measurable quantity. The charge possessed by an object is often expressed using the scientific unit known as the Coulomb. Just as mass is measured in grams or kilograms, charge is measured in units of Coulombs (abbreviated C). Because one Coulomb of charge is an abnormally large quantity of charge, the units of microCoulombs (µC) or nanoCoulombs (nC) are more commonly used as the unit of measurement of charge. To illustrate the magnitude of 1 Coulomb, an object would need an excess of 6.25 x 1018 electrons to have a total charge of -1 C. And of course an object with a shortage of 6.25 x 1018 electrons would have a total charge of +1 C.
The charge on a single electron is -1.6 x 10-19 Coulomb. The charge on a single proton is +1.6 x 10-19Coulomb. The quantity of charge on an object reflects the amount of imbalance between electrons and protons on that object. Thus, to determine the total charge of a positively charged object (an object with an excess of protons), one must subtract the total number of electrons from the total number of protons. This operation yields the number of excess protons. Since a single proton contributes a charge of +1.6 x 10-19 Coulomb to the overall charge of an atom, the total charge can be computed by multiplying the number of excess protons by +1.6 x 10-19 Coulomb. A similar process is used to determine the total charge of a negatively charged object (an object with an excess of electrons), except that the number of protons is first subtracted from the number of electrons.
This principle is illustrated in the following table.
| Object | # of Excess Protons/Electrons | Quantity and Kind of Charge (Q) on Object in Coulombs (C) |
| A | 1 x 106 excess electrons | -1.6 x 10-13 C |
| B | 1 x 106 excess protons | +1.6 x 10-13 C |
| C | 2 x 1010 excess electrons | -3.2 x 10-9 C |
| D | 3.5 x 108 excess protons | +5.6 x 10-11 C |
| E | 4.67 x 1010 excess electrons | -7.5 x 10-9 C |
In conclusion, an electrically neutral object is an object that has a balance of protons and electrons. In contrast, a charged object has an imbalance of protons and electrons. Determining the quantity of charge on such an object involves a counting process; the total number of electrons and protons are compared to determine the difference between the number of protons and electrons. This difference is multiplied by 1.6 x 10-19 Coulombs to determine the overall quantity of charge on the object. The type of charge (positive or negative) is determined by whether the protons or the electrons are in excess.
Check Your Understanding
Use your understanding of charge to answer the following questions. When finished, click the button to view the answers.
1. TRUE or FALSE: An object that is positively charged contains all protons and no electrons.
See Answer

Answer: False
Positively charged objects have electrons; they simply possess more protons than electrons.
2. TRUE or FALSE: An object that is negatively charged could contain only electrons with no accompanying protons.
See Answer

Answer: False
Negatively charged objects have protons; it’s just their number of electrons is greater than their number of protons.
3. TRUE or FALSE: An object that is electrically neutral contains only neutrons.
See Answer

Answer: False
Electrically neutral atoms simply possess the same number of electrons as protons. This gives the objects a balance of both type of charge.
4. Identify the following particles as being charged or uncharged. If charged, indicate whether they are charged positively or negatively. (n = neutron, p = proton, e = electron)

See Answer

Answers:
a. Charged Negatively
There are 11 electrons and 10 protons. This results in an imbalance of charge. With more electrons than protons, the particle is negatively charged.
b. Uncharged
There are 11 electrons and 11 protons. This results in a balance of charge. This particle is neutral or uncharged.
c. Charged Positively
There are 18 electrons and 20 protons. This results in an imbalance of charge. With more protons than electrons, the particele is positively charged.
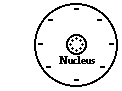 5. Consider the graphic at the right of a neutral oxygen atom.
5. Consider the graphic at the right of a neutral oxygen atom.
a. Explain what must happen in order for the oxygen atom to become negatively charged.
b. Explain what must happen in order for the oxygen atom to become positively charged.
See Answer

Answers: a. Gain electrons AND b. Lose electrons
Protons are tightly bound in the nucleus and can be neither gained nor loss. So any change in the charge of an atom is due to changes in its electron count. If a neutral atom gains electrons, then it will become negatively charged. If a neutral atom loses electrons, then it become positively charged.
6. Determine the quantity and type of charge on an object that has 3.62 x 1012 more protons than electrons.
See Answer

Answer: +5.8 x 10^-7 Coulombs (rounded)
To determine the charge on an object, determine the number of excess protons or excess electrons. Multiply the excess by the charge of an electron or the charge of a proton – 1.6 x 10-19 C. Finally, adjust the sign of the object to + or -.
7. Complete the following statements:
After some rather exhausting counting (and a rather tall tale), a physics teacher determines that a very small sample of an object contains …
a. … 8.25749 x 1017 protons and 5.26 x 1014 electrons; the charge on this object is ____ Coulombs.
b. … 3.12 x 1014 protons and 4.5488 x 1016 electrons; the charge on this object is ____ Coulombs.
c. … 2.40277 x 1019 protons and 9.88 x 1016 electrons; the charge on this object is ____ Coulombs.
d. … 2.6325 x 1015 protons and 2.6325 x 1015 electrons; the charge on this object is ____ Coulombs.
See Answer

Method: Subtract the smaller number from the larger number. (This would be based upon the exponent.) Then multiply the difference by the charge of a proton or electron – 1.6 x 10-19 C.
Answers:
a. 0.132 C
b. 0.00723 C (7.23 x 10-3 C)
c. 3.83 C
d. 0 Coulombs
(Someone could make a stronger case for the answer “impossible to tell” since the proton and electron count is not very precise. Given how the numbers are expressed, it is possible that there are 1021 more protons than electrons or 67439 more electrons than protons or … . But this is another topic.)
8. The amount of charge carried by a lightning bolt is estimated at 10 Coulombs. What quantity of excess electrons is carried by the lightning bolt?
See Answer

Answer: 6.25 x 1019 electrons
Multiply the charge in Coulombs (10 C) by the conversion factor: (1 electron) / (1.6 x 10-19 C). The units of C cancel; the answer is in electrons.
9. Respond to the following student statement:
“A positively charged object is an object that has an excess of positive electrons.”
See Answer

“I’ll bet you 20 bucks you’re wrong.” or “No Way!” or …
Electrons are not positively charged. Positively charged objects have an excess of protons (which are positively charged).