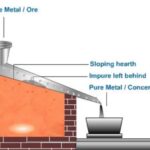
Metals occur in nature sometimes in free or native state, but most of these occur in nature in the form of chemical combination, i.e., in the form of their stable compounds which are associated with gangue or matrix. The earthy impurities such as sand, clay, rocks etc. associatedwith ore are collectively known as gangue or matrix. Thus a large number of metals in nature occur in the combined form along with other elements, but some metals, such as Ag, Au, Pt etc. occur in the native form (metallic state) in some regions. Ag occurs in native (or free) aswell as in the form of compounds. The natural material in which the metal or their compounds occur in the earth is known as mineral. Thus a mineral is a naturally occurring material present in earth’s crust which contains metal in the native (or free state) or in combined state.
A mineral may be a single compound or a complex mixture of various compounds. When a mineral contains sufficient amount of a metal in combined state, from which it can be readily and profitably separated on commercial scale, then the mineral is said to be an ore of the metal. Thus all ores are minerals, but all minerals are not ores. A mineral from which a metal can be profitably extracted is called an ore. For example, clay (Al2O3 2 SiO2 2 H2O) and bauxite (Al2 O3 2H2O) are two minerals of aluminium, but aluminium, can be profitably extracted only from bauxite and not from the clay. Hence bauxite is an ore of aluminium, while clay is a mineral. The biggest source of metal is the earth’s crust and the process of taking out the ores from the earth crust is called mining.
In the combined state ores are generally found in the form of oxides, sulphides, carbonates, sulphates, chlorides and silicates.
Ore Ore or Composition Metal
Mineral Present
Oxide ores
Bauxite Al2O3 .2H2O Al
Cuprite Cu2O Cu
Haematite Fe2O3 Fe
Zincite ZnO Zn
Tinstone or SnO2 Sn
Casseterite
Pyrolusite MnO2 Mn
Pitch Blende U3O8 U
Sulphide ores
Copper Cu2S, Fe2S3 or Cu2S . FeS2 Cu
Pyrites
Copper Cu2S Cu
Glance
Zinc Blende ZnS Zn
Cinnabar HgS Hg
Galena PbS Pb
Argentite or Ag2S Ag
Silver
Glance
Carbonate ores
Magnesite MgCO3 Mg
Dolomite CaCO3.MgCO3 Mg
Calamine ZnCO3 Zn
Malachite CuCO3.Cu(OH)2 Cu
Limestone CaCO3 Ca
Halide ores
Rock Salt NaCl Na
Carnallite KCl.MgCl2.6H2O Mg
Horn Silver AgCl Ag
Sylvine KCl K
Cryolite 3 NaF.AlF3 or Na3AlF6 Al
Sulphate ores
Epsom Salt MgSO4.7H2O Mg
Gypsum CaSO4.2H2O Ca
Barytes BaSO4 Ba
Anglesite PbSO4 Pb
Silicate ores
Asbestos CaSiO3.3MgSiO3 Mg
Felspar K2O.Al2O3.6SiO2 or Al
KAlSi3O8
Mica K2O.3Al2O3.6SiO22H2O Al
Phosphate ores
Phosphorite Ca3(PO4)2 P