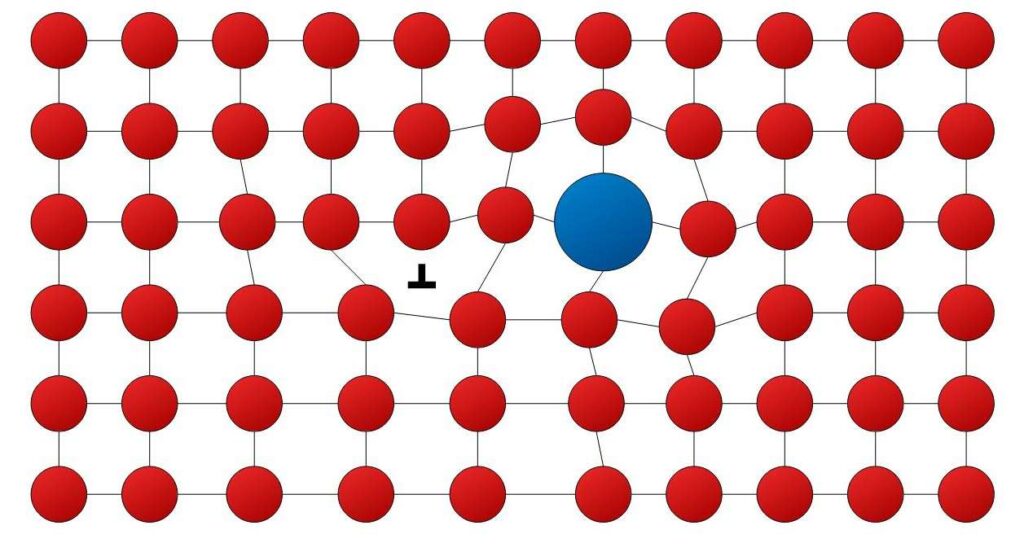
Adding another element that goes into interstitial or substitutional positions in a solution increases strength. The impurity atoms cause lattice strain (Figs. 7.17 and 7.18) which can “anchor” dislocations. This occurs when the strain caused by the alloying element compensates that of the dislocation, thus achieving a state of low potential energy. It costs strain energy for the dislocation to move away from this state (which is like a potential well). The scarcity of energy at low temperatures is why slip is hindered. Pure metals are almost always softer than their alloys.
Posted inMaterial Science
Solid-Solution Strengthening
Posted by
 admin
No Comments
admin
No Comments
Suresh Kumar is a passionate mechanical engineer with deep expertise in design, thermodynamics, manufacturing, and automation. With years of experience in the industry, they simplify complex engineering principles into practical insights for students, professionals, and enthusiasts. This blog serves as a hub for exploring cutting-edge innovations, fundamental concepts, and real-world applications in mechanical engineering.
Post navigation
Previous Post
 Strengthening by Grain Size Reduction
Strengthening by Grain Size ReductionNext Post
Strain Hardening 
