1Introduction
Past research shows that legacy persistent organic pollutants (POPs) such as dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), and hexachlorocyclohexanes (HCHs) pose substantial problems related to environmental and ecosystem health on a global scale [1–5]. POPs can be transported over very long distances, biomagnify in food webs, and cause adverse health effects in high trophic level species such as birds and marine mammals. Cold regions that are typically isolated from anthropogenic activity, such as the Arctic and the Antarctic, are particularly vulnerable to POPs because of the global distillation phenomenon, which causes many pollutants to concentrate in these regions [6, 7]. The Arctic Monitoring Assessment Program (AMAP), in association with the United Nations Environment Programme (UNEP) Stockholm Convention on Persistent Organic Pollutants, has played a key role in documenting the fate, transport, and effects of these pollutants in the Arctic, and has promoted global initiatives to monitor, manage, and control these substances [6, 8]. Despite enhanced understanding of POP contamination in the Arctic, limited information exists on the state of pollution in Antarctic food webs. Researchers have identified a lack of comparative data between the polar regions of the world, where many efforts have been directed toward understanding POP contamination in the high latitudes of the Northern Hemisphere such as the Canadian Arctic and Greenland [8–12]. Ongoing research has identified emerging contaminants of concern, including perfluorinated contaminants (PFCs), which are expected to pose significant risks to the environment and wildlife, particularly in the Arctic and the Antarctic [13–15]. Although PFCs have been detected in some Antarctic ecosystems and biota, the environmental transport and bioaccumulation patterns of PFCs, mainly perfluoroalkyl acids (PFAAs) such as perfluorinated carboxylates (PFCAs) and perfluorinated sulfonates (PFSAs), remain relatively unexplored within Antarctica. PFCs are highly fluorinated anthropogenic compounds, often utilized as repelling agents, with applications including coatings for paper or food packaging and textiles, industrial surfactants, insecticides, and historically, aqueous film-forming foams [16,17]. Due to their widespread use, PFCs are now considered environmentally ubiquitous substances, found in all areas around the world. In response, numerous measures have been taken to reduce the adverse impacts of PFCs on local and global scales [8]. PFCs are extremely persistent, can travel long distances (predominantly via ocean currents), bioaccumulate in food webs, and achieve highest concentrations in marine mammals and birds. PFCs are of particular ecological and toxicological concern due to their tendency to biomagnify in food webs and cause adverse health effects, including reproductive damage, immunotoxicity, and hepatotoxicity [18]. Of further interest is the unique physicochemical nature of PFCs. Whereas many legacy POPs are lipophilic and therefore accumulate in fatty tissues, PFCs tend to accumulate primarily in protein-rich tissues, such as the liver. Two PFAAs, perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), represent the most commonly investigated PFCs of significant risk to wildlife and humans due to their ubiquitous nature, global fate and transport, high biomagnification potential, and toxicity risks, especially in aquatic and marine food webs of the northern hemisphere and Arctic [18–21]. Phase out programs designed to eliminate the production of PFOS were established for some regions in the early 2000s, followed by the addition of PFOS to the list of restricted POPs under the Stockholm Convention on Persistent Organic Pollutants in 2009 [8]. Despite these initiatives, production of PFOS, PFOA, and several other PFAAs still take place around the world, including several developing countries [22–25]. One of the priority actions under the Antarctic Treaty is the assessment and monitoring of POPs, including PFCs, in Antarctica. Considering that assessments of PFCs in the Antarctic are limited, questions linger about the long-range environmental transport of these substances to the Southern Hemisphere, and the capacity of these substances to bioaccumulate in Antarctic food webs.
PFCs have been detected in various Antarctic environmental media and biota, typically in the pg/g to ng/g range, though many samples return nondetectable levels or levels below the minimum level of quantification [9]. Recent studies show that levels of many PFCs in Arctic environments have been increasing, with concentrations of several PFCs equivalent to or surpassing that of DDT, PCBs, PBDEs, and other organochlorine pesticides [19]. Similar patterns are anticipated for PFCs in Antarctic environments as they are continuously delivered from other geographic locations via long-range transport. Although some PFCs are already categorized as POPs, the majority of these substances are not subject to global or local controls. To ensure that potential impacts of pollutants on Antarctic wildlife are considered in the global environmental agenda and throughout negotiations on commercial chemical production and use, it is important that a high-quality research program is developed on the fate and effects of contaminants in Antarctic ecosystems and wildlife. As part of an ongoing scientific initiative and collaboration between the Ecuadorian Antarctic Institute (INAE), Simon Fraser University (Canada), the Institute of Ocean Sciences (IOS, Fisheries and Oceans Canada, DFO), and the Escuela Superior Politecnica del Litoral (Ecuador), a study to investigate and monitor PFCs was initiated in Peninsula Antarctica around the surrounding areas of the Ecuadorian Station “Pedro Vicente Maldonado” during the 2009 Ecuadorian–Antarctic expedition. In this chapter, we provide one of the primary findings on PFCs in sediments and biotic matrices, including lichens as well as feces and feathers from the southern giant petrel (Macronectes giganteus) and gentoo penguin (Pygocelis papua), and evaluate the use of noninvasive techniques to monitor emerging organic contaminant of concern in the Antarctic environment.
Materials and methods
STUDY AREA AND SAMPLING
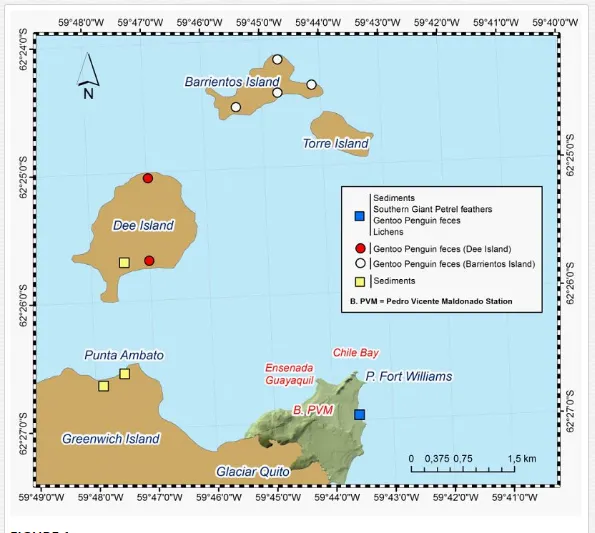
The Ecuadorian Research Station “Pedro Vicente Maldonado” (Maldonado Station, hereafter) is located at Fort William Point, Greenwich Island (62°31’S; 59°46’W; Figure 1). The study area encompassed the Barrientos Island (62°24’01″S; 59°43’ 52″W), Dee Island (62º25’48.5″ S; 59º47’69.6″ W), Punta Ambato (62º26’33” S; 59º47’28.8″ W), and the surroundings of the Maldonado Station (62º27’59”S; 59º43’32.5”W), as illustrated in Figure 1. Sampling was conducted using three tracks established by the Maldonado Station to access the coastline of Fort Williams, which enclose two sampling zones: Ensenada Guayaquil and Bahia Chile. These sectors are only used by technical and military personnel that work at the Station and visiting scientists that come to the island for research purposes. Barrientos Island is used principally as a tourist stopover for cruise ships where tourists land and walk around the island for bird-watching. In Dee Island and Punta Ambato, sampling was deployed around the coastline. All sampling was done during the Austral summer and seabird breeding seasons of 2009. The collection of abiotic and biotic samples is described as follows.
SEDIMENTS
Sediment samples were collected from three locations in the Antarctic Peninsula including Dee Island (n = 1 site), Maldonado Station (n = 2 sites), and Punta Ambato (n = 2 sites) (Figure 1). Sediment samples were directly collected using 100 mL centrifugation tubes, stored at < 4°C until transportation to the laboratory in Canada.
SEABIRDS
Gentoo penguins (Pygoscelis papua) and southern giant petrels (Macronectes giganteus), two species of seabirds that inhabit the Antarctic Peninsula, were identified as potential bio-indicators of PFCs contamination. The main reason for selecting seabirds is based on studies showing that bird populations are most affected by contaminants, specifically POPs, among wildlife species (see [26] for a review). Bird species have the greatest capacity to biomagnify chemicals because of their highly energy-efficient metabolic system and also because of their high trophic position within the food web. Bird populations are therefore often at high risk from bioaccumulative substances, and can act as the “canary in the coal mine” for the larger Antarctic ecosystem. In this context, we conducted a noninvasive sampling technique to minimize or completely avoid the impacts of lethal or invasive sampling on the local bird populations. Sampling focuses on the collection of shed/molted feathers and excreted fecal matter from nesting sites. Because of the very high affinity of PFCs for protein, feathers are good noninvasive sampling media for PFCS, as they consist mainly of protein matter (i.e., keratin, a high molecular weight protein). Feathers have also been used to successfully monitor mercury in seabird populations such as brown skuas, Catharacta lonnbergi, chinstrap penguins, Pygoscelis antarctica, and gentoo penguins, P. papua), in our study area [27], as well as PFCs in the feathers of aquatic and marine birds, including grey heron (Ardea cinerea) and herring gull, (Larus argentatus) from the Northern Hemisphere [28]. Fecal matter is known to contain some of the highest concentration of contaminants due to the gastrointestinal magnification that occurs in the intestinal tract of consumer organisms. In addition, the contaminant concentrations in fecal matter are related to compounds absorbed by the organism, such that they can provide a measure of accumulated concentrations. The low capacity to migrate to the gaseous phase (i.e., air) and high octanol-air partition coefficient (KOA) of the analytes (Table 1) cause minimal losses of the contaminants from feces or feathers to the air after feathers or fecal matter have been dropped. This means that the concentrations of the chosen analytes can remain a measure of bird exposure levels long after the feces have been excreted or feathers have been shed. Molted feathers were collected randomly in and around nests and colonies of petrels surrounding the Maldonado’s Station and stored in ziploc-type plastic bags (n = 5). Only one bag of feather samples for gentoo penguin was collected from the Maldonado’s Station. Fecal matter samples from gentoo penguins were collected from nesting sites and colonies around the Maldonado’s Station (n = 9), Barriento Island (n = 7), and Dee Island (n = 3). All feces samples were placed into 20 mL glass vials. Both feather and fecal samples were stored in coolers and transported by airplane with dry ice (–20°C) until transportation to the lab in Canada.
LICHENS
Lichen (Usnea aurantiaco-atra) samples (n = 5) were collected from rocky areas around the surroundings of the Maldonado’s Station and wrapped with clean, sterile aluminum foil and stored in ziploc plastic bags until further transportation to the lab. The rationale to select lichens is based on the premise that this biological matrix can be used as a potential monitor and indicator of global atmospheric transport of some PFCs to the Antarctic Peninsula.
PFC PHYSICAL – CHEMICAL PROPERTIES
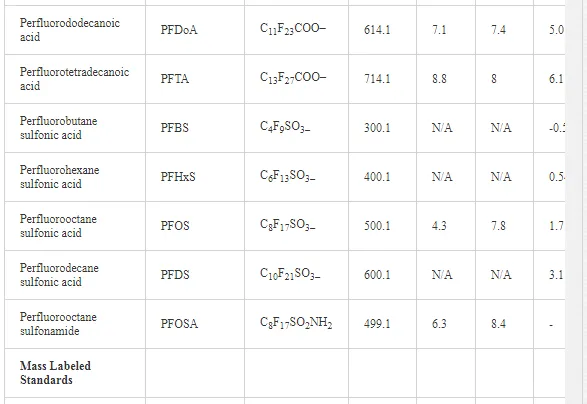
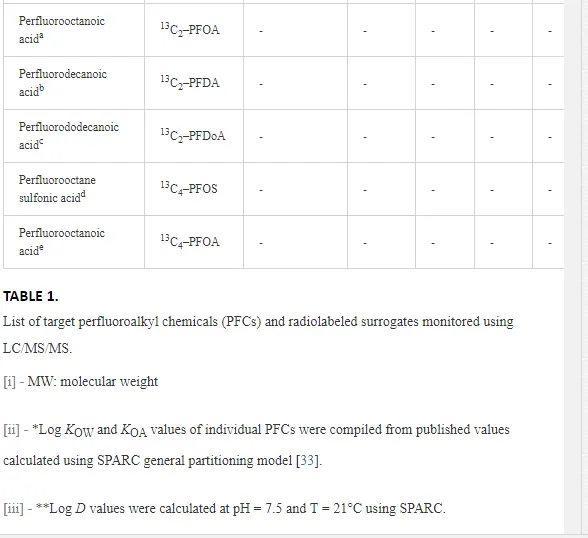
Table 1 summarizes the compiled physical–chemical properties for the various PFCs studied, including molecular weights (MW), log octanol–water partition coefficients (log KOW), log octanol–air partition coefficients (log KOA), and log D values. Because the physicochemical properties of PFCs are considerably different from that of many other legacy POPs (i.e., they can be ionized at environmentally relevant pH), it is important to recognize that relationships applicable to other POPs may be less relevant when applied to PFCs and other ionizable compounds. For instance, many organic compounds of concern, including numerous agricultural and pharmaceutical compounds, are lipophilic in nature, and will tend to accumulate in fatty tissues [3]. The octanol–water partition coefficient (KOW) has become a common property used to describe the tendency of a substance to partition into lipid, as the behavior of octanol and lipid are quite similar. Octanol thus serves as a suitable surrogate for lipid, particularly within predictive bioaccumulation models [29]. However, KOW describes the lipophilicity of neutral compounds, and is not necessarily applicable to ionizable organic compounds (IOCs) such as PFCs, where the measure of lipophilicity is pH-dependent [30]. Many PFCs are almost completely ionized at environmentally relevant pH [31]. A more applicable indicator for predicting the lipophilicity of ionizable substances is log D, where both the neutral and the ionic species of the compound are accounted for [30].
PFC ANALYSIS: EXTRACTION AND QUANTIFICATION
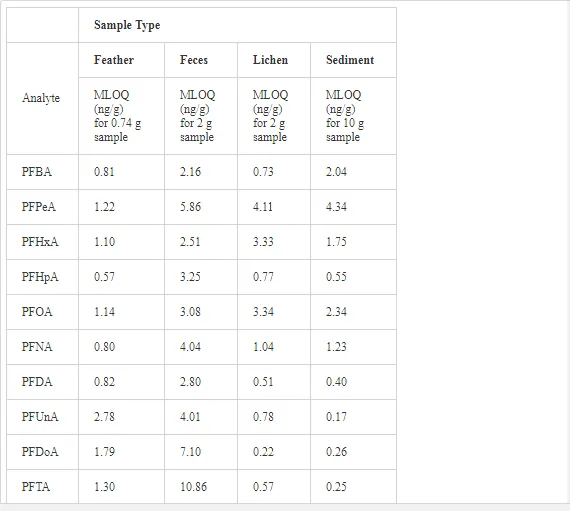
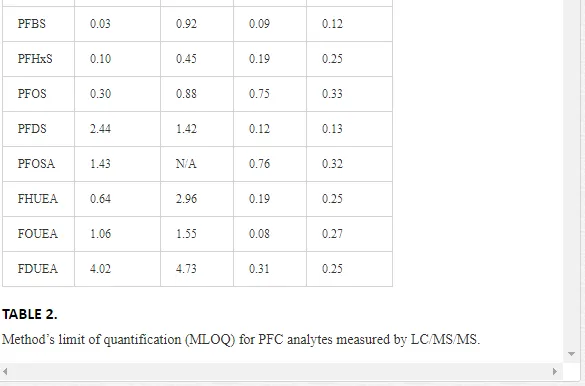
Sediment and biological samples were extracted and analyzed at the Institute of Ocean Sciences (IOS), Fisheries and Oceans Canada (DFO), Sidney, British Columbia, Canada. PFC concentrations were analyzed by liquid chromatography tandem mass spectrometry with double mass detectors (LC/MS/MS), as described elsewhere [19]. Analyte concentrations were determined with respect to the mass labeled quantification and internal standards using isotope dilution method. Fifteen PFCs were examined in this study (Table 1). High purity (>95%) analytical standards, including perfluorobutane sulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS), PFOS, perfluorobutanoic acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid (PFDoA), perfluorotetradecanoic acid (PFTA), and perfluorooctanesulfoamide (PFOSA), were used. Mass-labeled internal standards included six PFCs (13C2 PFOA, 13C2 PFDA, 13C2 PFDoA, and 13C4 PFOS, 13C4-PFOA). Calibration curves were constructed from the analysis of calibration standard solutions (range 0.08–5.0 ng/mL). Various calibration standards and standard additions were prepared and used as quality assurance/quality control (QA/QC). QA/QC measures included initial method validation work, consisting of analyte recovery experiments of native PFCs in clean sediments and biota. The method of detection limit (MDL) was set equal to the concentration of the method’s level of quantification (MLOQ) for samples and subtracted from quantified concentrations of each analyte (Table 2). Only corrected data above the MLOQ are reported in this work. Concentrations of PFCs were expressed on a wet weight basis (ng/g ww). Extraction methods are briefly described as follows.
SEDIMENT
Sediment samples (≈ 10 g wet weight) were added to 50 mL polypropylene centrifuge tubes and spiked with internal surrogate spiking solution (360 ng of 13C2 PFOA, 120 ng of 13C2 PFDA, 120 ng of 13C2PFDoA, and 120 ng of 13C4 PFOS; Table 1). After 20 min, 10 mL of 0.1% acetic acid in MeOH was added, and samples were extracted on a shaker table for 16 h. After extraction and centrifugation, 1 mL was pipetted into 1.5 mL Ependorf vial containing 25 mg of activated carbon. Then the vial was subject to centrifugation for 30 min at 14,000 rpm; 300 μL of supernatant was taken and combined with 300 μL of water and 50 μL of 20 ppb of recovery standard and centrifuged again for 15 min at 14,000 rpm. Then, 300 μL of supernatant was used for LC/MS/MS analysis (i.e., injection volume=100 μL for LC/MS/MS).
FEATHERS
Approximately 0.74 g of feather was weighed, and then homogenized by adding first HNO3 (e.g., 4 × 0.9 mL, 2 × 0.9 mL) with a series of vortexing steps until the whole particulates completely disappeared within 3 h. Samples were set up for digestion at room temperature (RT) for 12 h. Afterwards, 15 mL of 5 M NaOH prepared in water was added to samples and shaken on a shaker table for 5 min. The pH was measured to ensure the sample was acidic enough (i.e., pH~3–4) prior to direct injection in LC/MS/MS (large volume injection). After neutralization and extraction with 2.5 mL MeOH for a total volume 27.5 mL, ion suppression was found from recoveries; therefore, additional dilution (10×) was done until ion suppression was reduced (i.e., injection volume = 200 μL).
FECES
Penguin fecal matter (~0.65 g of feces) was weighed and homogenized with HNO3 (e.g., 4 × 0.9 mL, 2 × 0.9 mL and vortexing). Samples were set at RT for digestion during 12 h. After digestion, samples were neutralized to pH equal to 3.2–4.2, brought up to 50 mL and centrifuged at 6000 rpm for 25 min; 100 μL of recovery standard (13C4-PFOA) was added to an aliquot of 400 μL and injected into LC/MS/MS (i.e., injection volume= 200 μL).
LICHEN
Lichen (2 g) was extracted based on the methodology described in reference [32]. After extraction, 4 mL of solution was blown down to 2 mL, followed by collecting 1 mL aliquot and added into 1.5 mL Ependorf vial containing 25 mg of activated carbon. The vial was subject to centrifugation for 30 min at 14,000 rpm and 300 μL of supernatant was obtained and combined with 300 μL water and 50 μL of 20 ppb recovery standard and centrifuged for 15 min at 14,000 rpm. Then, 300 μL of supernatant was used for LC/MS/MS analysis (i.e., injection volume=100 μL).


Result and discussion
PFC CONCENTRATIONS
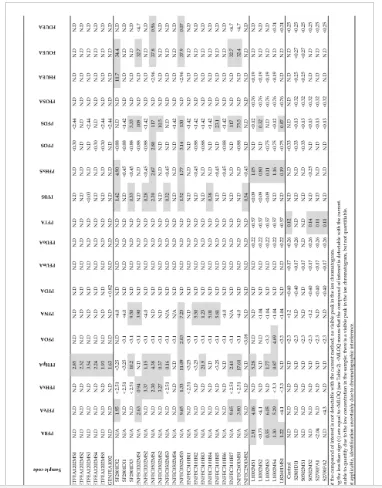
Several PFC compounds showed concentrations above the MLOQ, as shown in Table 3. Perfluorotetradecanoic acid (PFTA), a chemical with a high KOW and high KOA that will persist for decades in humans, was measured in 60% of sediment samples, but undetected or below the MLOQ in lichens, feces, and feathers. Perfluoroheptanoic acid (PFHpA) was detected in all seabird feather samples (range = 1.60−2.85 ww ng/g; Table 3), and in 47% of penguin feces, ranging 0.37−22 ng/g ww. All lichen samples exhibited concentrations of perfluorohexanesulfonate (PFHxS), ranging 0.20−1.20 ng/g ww, while perfluorobutyric acid (PFBA), perfluoro-n-pentanoic acid (PFPeA), and PFHpA were measured in 80%, 60%, and 60% of lichen samples, respectively. PFOA and PFOS were not quantified in most samples (i.e., < MLOQ or ND; Table 3), except for the detection of PFOS in two penguin feces samples (2.8 and 3.14 ng/g ww), and PFOA in a single fecal sample (2.0 ng/g ww) and lichen (4.7 ng/g ww). The lack of PFOS and PFHxS detection in Antarctic seabird feathers contrasts with the levels of PFOS and PFHxS found in feathers of grey herons (PFOS: 247 ng/g dw; PFHxS: ≈ 20 ng/g dw) and herring gulls (PFOS: 79 ng/g dw; PFHxS: > 30 ng/g dw) from the Northern Hemisphere (Flanders, Belgium) [28]. However, the absence of PFOA in our feather samples is consistent with the lack of detection of this compound in bird feathers from the same region [28]. For comparison purposes, the PFOA concentration detected in a sample of gentoo penguin feces was 14 times lower than the PFOA concentration (28.2 ng/g ww) detected in a single herring gull liver sample from Belgium [28]. Despite samples from other parts of the world that indicate a continued increase or no change in PFOS levels following the 2002 phase-out [34–37], a fast decline in PFOS concentrations has been observed in wildlife over the past decade [38,39]. PFDA, PFUnA, PFDoA, and PFOSA were not detected (ND) or < MDL. Except for the compound PFHpA, lack of detection of most analytes in samples and small sample sizes preclude undertaking robust statistical analyses for multisite or/and inter-species comparisons.
PFC PATTERNS
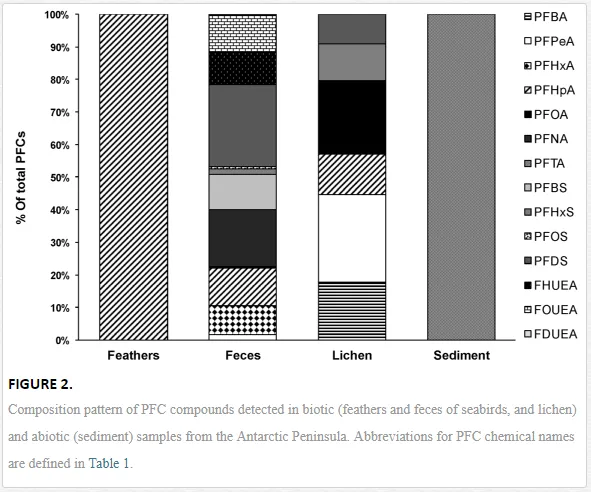
Figure 2 shows the composition of PFCs observed in biotic and abiotic samples. PFHpA was the only compound detected in feathers of both petrels and penguins, accounting for 100% of total PFCs, while PFTA was equal to 100% of PFCs in sediment samples (Figure 2). PFHpA was also found in feces and lichen samples making up 24.5% and 23% of total PFCs, respectively. PFDS contributed to 54% and 17% of PFCs in feces and lichens, contrasting with PFPeA and PFHxS, which accounted for 3.4% and 50%, and for 4% and 21% of PFCs in feces and lichen samples, respectively. PFNA accounted for 38% of the PFCs in feces. These patterns clearly show that both perfluorinated sulfonates (PFSAs) and carboxylates (PFCAs) exhibit different fractions in seabirds, reflecting the potential role of biotransformation in shaping the accumulation of these compounds
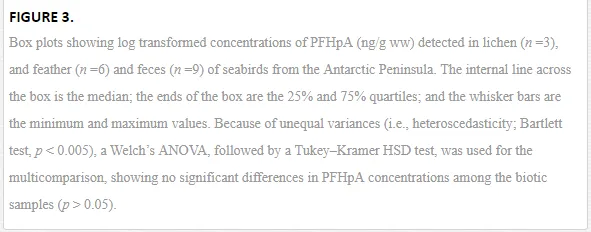
BIOACCUMULATION OF PFCS
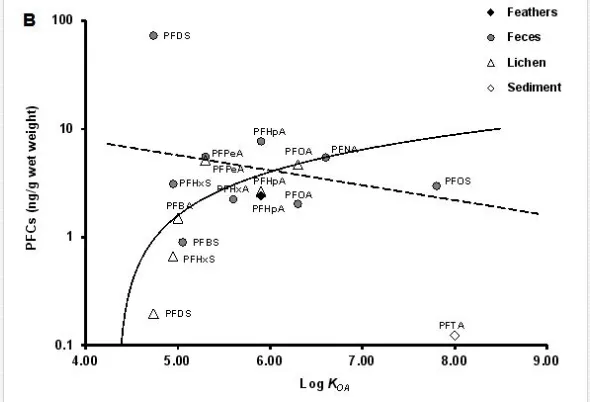
The biomagnification factor (BMF) [40] for PFHpA (i.e., BMF = C B/CD, where CB is the PFHpA concentration detected in the predator, the giant petrel, and CD is the PFHpA concentration observed in the diet/prey, gentoo penguin) was calculated using feather concentrations, as this was the only PFC compound readily detected in 100% of feathers samples. Hence, the concentrations of PFHpA in the petrel feathers (i.e., mean ±SD = 2.6 ± 0.60 ng/g ww; n = 5) and that of the penguin (1.60 ng/g ww; n=1; Table 3) were used as surrogates for concentrations in the tissues of the whole organism, assuming that the birds had been exposed to the compound for a sufficiently long time to allow the concentrations to reach steady state [40]. The criterion applied to indicate that PFHpA was biomagnified in petrels was a BMF > 1, such that a BMF greater than 1 indicates that the chemical is a bioaccumulative substance [41]. Here, we found that the BMF was close to 2 (i.e., 1.6), indicating that PFHpA biomagnifies in petrels. Although the concentrations of PFHpA in feces appear to be relatively higher than the concentrations found in lichen and feathers, comparisons of the PFHpA concentrations among biota samples show lack of significant differences (Welch’s ANOVA, p > 0.05; Tukey–Kramer HSD (honest significant difference) test, p >0.05), as shown in Figure 3. To further illustrate the behavior of PFC concentrations in these samples, detected PFC compounds were plotted as a function of log D and log KOA, as shown in Figure 4. The majority of PFCs concentrations observed in biotic samples (i.e., feces and lichens) fall within log D values between 0 and 3, as seen in Figure 4A. While concentrations of PFCs tend to increase with increasing log D values from log D of 0 to log D of 3 in feces, PFC concentrations appear to decrease as the log D increases within the same range of log D values in lichens (Figure 4A). This observation may be an indication that both ionized and unionized forms of PFC compounds with low log D values (i.e., PFBA, PFPeA, PFHxA, PFHxS, PFHpA, PFOS, PFOA, PFNA, PFDS) are present in some organisms residing in this region and prone to potential transportation by oceanic currents (e.g., Antarctic Circulation Current) from either continental/regional or local sources (i.e., international military bases and research stations) to the Antarctic Peninsula. Similarly, most PFCs concentrations observed in these samples, especially in lichens, fall within log KOA values of 5.0 and 6.5 (Figure 4B). Although concentrations for some PFC compounds show a tendency to decrease with increasing log KOA in feces (i.e., PFBS, PFHxS, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFOS), concentrations for similar PFCs seem to increase as log KOAincreases in lichens (i.e., PFDS, PFHxS, PFBA, PFPeA, PFHpA, PFOA), as seen in Figure 4B. These trends may support the notion that low molecular weight compounds (e.g., 214-414 g/mol) with low log KOA are likely to be subject to long-range atmospheric transport and potentially reaching the region, where these compounds accumulate in biotic compartments, mainly in natural air samplers such as lichens and secondary in air-breathing organisms such as seabirds (petrels, penguins).



PFC HEALTH RISKS
Concentrations of PFCs detected in feathers and feces of the two seabird species studied here are well below the toxicological reference value (TRV) of PFOS (600 ng/g ww), calculated as an exposure threshold value for birds in nature, especially for apex avian predators [42]. This comparison indicates that gentoo penguins and petrels are not at risk by PFOS toxic effects.
TRANSPORT MECHANISMS, GLOBAL AND LOCAL SOURCES OF PFCS TO THE ANTARCTIC
There is still a degree of uncertainty surrounding the dominant pathway of PFC movement to the Antarctic, though researchers have highlighted two primary mechanisms generally accepted as the major modes of PFC transportation to the Antarctic: atmospheric and oceanic. Neutral, volatile precursor compounds, such as perfluorinated sulfonamide alcohols (FOSEs), perfluorooctane sulfonamides (FOSAs), and fluorotelomer alcohols (FTOHs), referred to as “flyers”, are capable of being delivered to the Antarctic via fast, direct transport of contaminated wind, as opposed to cold trapping, as is common for many legacy POPs [12, 43–45]. Following deposition, these compounds are degraded via oxidation to form ionic PFCs, including PFSAs and PFCAs [12, 44–48]. Evidence supporting this mechanism of travel includes measurements of FTOHs from Europe to the Antarctica showing declining concentrations in the atmosphere with increased distance from sources in the Northern Hemisphere [12]. Given the far distances PFCs must travel to reach the Antarctic, in combination with short atmospheric residence times (ranging on average from 10 to 50 days), the level of effectiveness associated with atmospheric delivery of PFCs is relatively low. Additionally, the yield of ionic PFCs produced via oxidation of precursor compounds once transported to the Antarctic is often low [10, 11, 43–45, 47]. It is therefore expected that most PFCs are delivered to the Antarctic in their ionic, water-soluble state via the oceans [12, 14, 49]. Oceanic transport functions on a slower time scale for the Antarctic (in the order of decades, compared to days or weeks for atmospheric transport) because of the circulation patterns of the Southern Ocean, protecting the Antarctic from immediate fluxes in PFC concentrations as they are released elsewhere in the world. As time progresses, however, contamination from oceanic sources is anticipated to increase [10, 11, 45]. Slow oceanic transport is cited as the reason for increasing PFC concentrations in the Arctic since the 1950s [14]. Models designed for Arctic research show that if oceanic transport to the Arctic ceased, the quantity of PFCs and their precursors delivered to the Arctic via atmospheric transport could not account for the concentrations measured in water, and thus marine transport is considered to be more important than atmospheric transport [15, 43]. It is also important to note that atmospheric and oceanic transport may be difficult or impossible to discern. For instance, PFCs found in the ocean are made up of three inputs: direct emissions to water, atmospheric deposition into water, and precursor compounds into water followed by degradation to ionic PFCs [14].
Among the compounds found in this study, PFHpA, PFBA, and PFPeA are byproducts of stain/grease-proof coatings on food packaging, couches, and carpets, while PFHxS was used in fire-fighting foams and carpet treatments and phased out of consumer products along with PFOS and PFOA by the major manufacturer (3M Co.) in the early 2000s due to health risks. While long-range atmospheric and oceanic transport of PFCs may partially explain the ubiquitous nature of these contaminants in the Antarctic Peninsula, military bases and infrastructure of nations established there may also be contributing sources of PFCs in Antarctic ecosystems. Atmospheric long-range transport of PFAAs as marine aerosols and degradation of PFCA and PFSA precursors such as low molecular weight FTOHs and acrylates/acids (FTAs) or perfluoroalkyl sulfonamids (FASA) and sulfonamido ethanols (FASE), which are more volatile and released to the atmosphere during fluoropolymer production processes, can be considered as other major pathways [11, 14, 44, 50, 51] to reach and deposit on the Antarctic Peninsula.
Additional and potential sources of PFAAs in the Antarctic Peninsula include aqueous film-forming foams (AFFF) and emissions of a current use insecticide, sulfluramid (N-ethyl perfluorooctane sulfonamide), to control leaf-cutting and fire ants in South America [20, 52; J. Benskin, pers. comm., June 2012). AFFF formulations have consisted of perfluoroalkyl sulfonates (PFHxS, PFOS, PFDS) and more recently, fluorotelomer sulfonamide-based surfactants. While these latter materials can degrade down to short-chain perfluoroalkylcarboxylates (typically C4, C5, C6 PFCAs), sulfluramid can degrade to PFOS, FOSA, and PFCAs by abiotic and/or biological processes [53, 54]. Sulfluramid is manufactured in Brazil (≈30 tons/year in 2007), and, in 2006, about 12 tons was exported to 13 other Central and South American countries [23, 55]. Because this insecticide is a semivolatile substance, it could be transported atmospherically to the Antarctic. Sulfluramid degradation products include PFOSA, PFOS, and potentially PFOA [52]. Despite high concentrations of PFOS, PFOA, and PFOSA measured off the Atlantic coast of South America (South Atlantic), increasing from Brazil to near Rio de la Plata (Argentina–Uruguay), attributed to the use of this substance [52], PFOS and PFOA were not detected in these Antarctic samples, with the exception of two feces samples and a lichen sample. This indicates that these two compounds have not yet fully reached the Antarctic Peninsula region, or local sources are not significant. The detection of several PFCA compounds in the present study is of particular importance as increasing trends of PFCA precursors (i.e., FTOHs) was observed in the Arctic with doubling times of 2.3–3.3 years from 2006 to 2012 [6].
Impact assessment, environmental management, and monitoring implications
The Ecuadorian Pedro Vicente Maldonado Scientific Station has been operated since 1988 shortly after Ecuador signed the Antarctic Treaty System (ATS) in 1987. In 1988, Ecuador became an associated member of the Scientific Committee for Antarctic Research (SCAR), and in November 1990 became a consultative member of the ATS [56, 57]. To accomplish this task, Ecuador fulfilled the Antarctic Treaty of “peaceful purposes” and “freedom of scientific investigation” [58]. The commitment to the protection of the Antarctic environment requires being in compliance with the Madrid Protocol, which since 1991 is the prime basis for environmental management of the Antarctic terrestrial and near‐shore environments. At the Maldonado Station, the Antarctic environmental management program deploys and integrates a range of generic and international tools, including environmental impact assessments (EIAs), monitoring of pollutants in the marine environment, species and habitat protection, following the environmental principles of the Madrid Protocol, and the administrative and procedural mechanisms of the Committee for Environmental Protection (CEP) [58]. The Ecuadorian Antarctic Institute (INAE) has established good environmental practices, and trained their staff and visitors with a conduct code according to the Madrid Protocol. During the 2010–2011 period, an EIA was performed by the INAE [59] to establish the baseline conditions of the military base and research station, including its area of influence, developing the Environmental Management Plan for the activities taken place at the Maldonado Station.
Considering that local activities and maritime traffic can pollute the surroundings of the Maldonado Station and Antarctic Peninsula, results from impact assessments and monitoring of water quality and potential contaminants have revealed the presence of other anthropogenic pollutants such as hydrocarbons and pesticides in the marine environment [59]. For instance, analyses of total hydrocarbons (THCs) were performed in water samples at the Guayaquil Bay, where concentrations ranged from 0.3 mg/L in sites near the Maldonado Station to 0.85 mg/L at Chile Bay [59]. Pesticide concentrations at several sites of Chile Bay revealed the presence of the organochlorine insecticides, including lindane or γ-hexachlorocyclohexane (γ-HCH) (i.e., 0.335 mg/L) and β-hexachlorocyclohexane (β-HCH or beta-BHC) (i.e., 0.00072 mg/L), as well as traces of the organophosphate malathion and the herbicide atrazine [59]. The long-range atmospheric transport associated with direct deposition or precipitation of volatile organic chemical is now recognized as a major pathway by which pesticides can be transported and deposited in surface waters and ice of Antarctica thousands of kilometers far from their sources [60–62]. Although relatively low concentrations of some PFCs were observed in biota and sediments samples of the remote western Antarctic Peninsula environment and local sources associated with scientific stations and military bases appear to not be significant sources of PFCs, this study gives further evidence of background concentrations around the Antarctic. Results from this study are consistent with research showing that volatile PFCs are subject to atmospheric long-range transport to remote regions, contributing to the contamination of persistent PFCA and PFSA compounds in the Antarctic [11, 44]. Despite the limited sampling and the need for replication to confirm the findings of this study, the biological (lichens, feathers, feces) and abiotic (sediments) samples assessed in this work can be used as environmental matrices to track the fate of PFCs at various temporal and spatial scales in Antarctica. Of particular importance is the detection of several PFC compounds in seabird feces and lichens, which can be used as matrices for noninvasive sampling and long-term monitoring programs of PFCs in the Antarctic Peninsula. Long-term air monitoring and sampling of volatile PFCs in the Antarctica Peninsula is also recommended to elucidate atmospheric sources to the Maldonado’s Station and surrounding environment.