Introduction
The ability of persistent organic pollutants (POPs) to spread through long distances and remain in the environment has resulted in their presence worldwide. They have a tendency to accumulate within the food chain and, as a result, pose a high risk to human and animal health. The risks caused by industrial POPs are best illustrated by polychlorinated biphenyls (PCBs), extensively used in a wide variety of applications from the early 20th century. PCBs became ubiquitous contaminants of various biotic and abiotic environments worldwide due to their massive and uncontrolled use in industry and agriculture [1, 2]. PCBs are highly persistent compounds in the environment, especially in aquatic sediments, which act as a stable reservoir from which PCBs can continue to be released over a long period of time, because of their low solubility in water and low volatility [3]. The involvement of PCBs in the food chain occurs through the incorporation of suspended particles in phytoplankton and zooplankton, at the base of the food chain. Bottom feeders and other aquatic organisms ingest, accumulate and pass PCBs upward in the food chain [4, 5]. Fish near the top of the aquatic food web have a relatively long life span and concentrate high amounts of PCBs [6]. The concentration of poorly metabolized chemicals accumulated in fish can thus reflect the degree of pollution in an aquatic system [7, 8]. Biomonitoring is a vital and rapidly growing field that uses several biological groups, such as phytoplankton, macrophytes, invertebrates and fish, as bioindicators [9]. This ecological methodology is increasingly used to assess the level of pollution in aquatic environments [10–13]. Numerous studies have also documented the close relationship between aquatic pollution and parasitism [e.g. 13, 14]. Certain parasitic organisms have the ability to concentrate high quantities of pollutants in their tissues and organs and thus can provide information about the chemical state of the environment [15, 16]. These studies have mainly focused on intestinal parasites, mostly acanthocephalans, as indicators of heavy-metal pollution [14, 17, 18], but data on organic pollutants, including PCBs and their bioaccumulation in parasitic organisms, are still scarce.
The Zemplínska šírava water reservoir (eastern Slovakia; Fig. 1) is one of the most PCB-contaminated sites in Europe [19, 20]. Large amounts of PCB compounds have been released to the reservoir from a chemical factory in the nearby town of Strážske without treatment (decontamination) or preventive measures, leading to heavy contamination of soil, superficial and underground water and subsequently food chains in this area [21]. The PCBs in the reservoir and the inflowing Šíravský canal thus belong to the so-called old environmental burdens, and their amount varies from tens to hundreds of milligrams per kilogram of sediment dry weight. At least 40, 000 tons of PCB-contaminated sediments are assumed to be still present in the reservoir, effluent canal and Laborec River [22]. Studies over the past 20 years have indicated that elevated concentrations of PCBs in the reservoir pose a potential hazard to human and aquatic health [19, 23].
Material and methods
STUDY AREA
The Zemplínska šírava reservoir (48°47′32″N; 22°0′42″E), one of the largest artificial water-storage sites in Slovakia, is located in the eastern part of the country (Fig. 1)
.

It was built between 1961 and 1965 for regulating flood overflows from the Laborec River and partly for irrigation purposes in the area. The reservoir is 11 km long, ca. 3.5 km wide and covers an area of 33 km². PCB compounds from the chemical factory in Strážske were released into the Strážsky (effluent) canal to the Laborec River and from there by the Šíravský (influent) canal to the Zemplínska šírava reservoir (Fig. 1). The reservoir has abundant ichthyofauna, represented predominantly by dense populations of common carp, Cyprinus carpio L.; freshwater bream, Abramis brama L.; pike-perch, Sander lucioperca L.; northern pike, Esox lucius L.; European perch, Perca fluviatilis L.; Wels catfish, Silurus glanis L. and goldfish, Carassius auratus L. The PCBs in fish were monitored twice, first in 2004 and then in 2009, at eight sites in the reservoir (Fig. 1).
FISH AND PARASITE COLLECTION
A total of 50 fish of nine species belonging to five families (Anguillidae, Cyprinidae, Esocidae, Percidae and Siluridae) were collected. The distribution and concentrations of PCBs were determined in predatory (P. fluviatilis, E. lucius, S. lucioperca and S. glanis) and non-predatory (C. carpio; the European eel, Anguilla anguilla; Ab. brama; C. auratus and the roach, Rutilus rutilus) species. The fishes were transported to the laboratory alive, weighed, measured and divided into feeding guilds [24, 25] (Table 1).
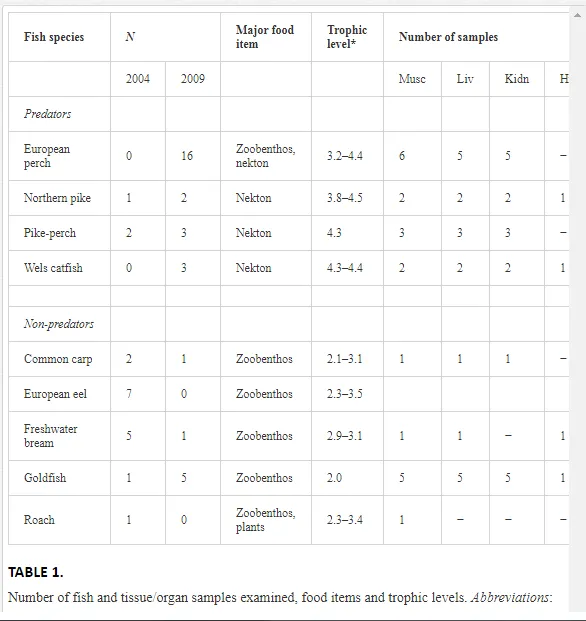
Only muscle tissues (19 samples) were collected in 2004. A more detailed study in 2009 focused on the tissue-specific distribution of PCBs to assess the temporal variations of PCBs in the reservoir. Samples of the liver (hepatopancreas in cyprinids), kidney, adipose tissue, hard roe, bone and brain were collected from 31 fish belonging to seven species [26]. Only perch were infected with parasites and so received special attention. Twelve of 16 perch in a single catch were small, and therefore the tissue/organ samples from these fish were pooled by the weight of the fish: up to 10 g (n=7) and 14–30 g (n=5). The four remaining fish weighed 60–130 g and were analysed separately. A total of 24 samples of muscles, livers, kidneys, adipose tissues and brains were examined for the presence of PCBs (Table 1). Screening of the digestive tracts for parasites using a stereomicroscope found only one helminth species, the acanthocephalan Acanthocephalus lucii. The two largest perch (>120 g) contained 50 and 19 acanthocephalans. The remaining fish were either free of acanthocephalans or their infections were low, mostly 1–2 acanthocephalans per fish in the 10–30 g group. Perch weighing 60 g contained a maximum of five parasites. Perch harbouring such low parasite burdens were considered as uninfected for the comparison of PCB accumulation in infected versus uninfected perch. The parasites were washed in saline, frozen and subsequently examined for PCBs. A total of 95 tissue/organ samples of all fish species (Table 1) and two samples of acanthocephalan parasites were analysed spectrophotometrically [27]. The scientific and common names of the fish match those in the FishBase database [25].
ANALYTICAL PROCEDURE
The extraction and clean-up of the samples followed the methods described by Himberg et al. and Fisher and Ballschmiter [28, 29], with slight modifications. Briefly, the fish and parasite samples were homogenized in anhydrous sodium sulfate and extracted with a mixture of petroleum ether (90%) and acetone (10%) using a separation funnel. The extracts were concentrated in a rotary evaporator and then purified using a Florisil chromatographic column according to STN EN 12393-2 (2001). The final extracts were analysed on a 6890 gas chromatograph (Hewlett-Packard) equipped with and electron capture detector. The HP-5 capillary column was 30 m in length, with an i.d. of 0.25. and having a film thickness of 0.25 µm. The chromatograph was operated at an injector temperature of 250°C and a detector temperature of 300°C and used helium as the carrier gas at a flow rate of 1.4 ml min–1. The following oven temperature program was used: start temperature 80°C for 1 min, 80–180°C at 30°C min–1 and maintained for 1 min, 185–205 °C at 6°C min–1 and maintained for 15 min and 205–290 °C at 20°C min–1 and maintained for 7.5 min. Individual PCB congeners were identified by their comparison with the retention times of known standards and qualified by comparing the peak areas to the appropriate peaks in the standard mixture (PCB Mix C-SCA-06). The extracts were injected under a splitless mode. The recovery rates of the PCBs in the spiked samples were 80–95%, whereas the detection limits were 1 μg kg–1 based on wet weight. Six PCB indicator congeners – PCB 28, 52, 101, 138, 153 and 180 – were analysed. All PCB concentrations in the biological samples are given in mg kg–1 lipid weight (lipid wt).
STATISTICAL ANALYSIS
The data obtained did not meet the requirements for parametric statistical tests (normality). So they were analysed non-parametrically or were log-transformed [ln (x + 1)] to satisfy the assumptions (normal distribution and homogeneity of variances) of analyses of variance (ANOVAs). Differences in PCB load between years and between fish tissues/organs and parasites were determined by t-tests. The effects of trophic strategy (predatory and non-predatory fish) and tissue/organ type on PCB levels were tested with two-way ANOVAs. Main-effect ANOVAs were used instead of factorial ANOVAs because no significant interactions were confirmed between the factors. The data were consequently divided into two groups, predatory and non-predatory fish, and were processed separately. The concentrations are expressed as mean ± SD (standard deviation). Differences in mean concentrations (mg kg–1 lipid wt) of the congeners and total PCBs in the fish tissues/organs (muscle, liver, kidney, brain, adipose tissue and bone for predatory/non-predatory fish) were tested with a non-parametric Kruskal-Wallis ANOVA with post-hoc multiple comparison. The analyses were performed in Statistica for Windows, version 9.0 [30].
Results and discussion
PCB DISTRIBUTION ASSOCIATED WITH FISH SPECIES AND FEEDING HABIT
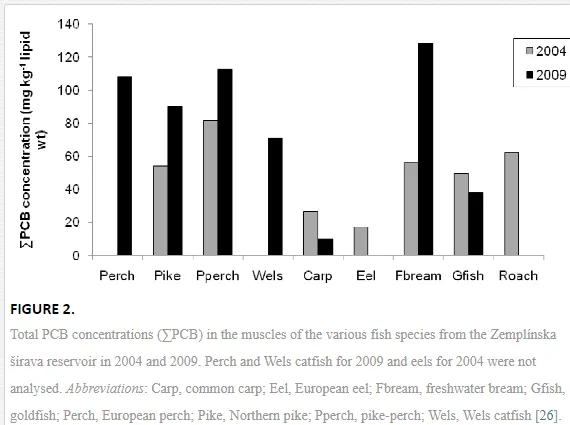
The total PCB concentrations (the sum of the six PCB congeners) in the muscle tissues varied greatly in the nine fish species collected in 2004 and 2009 from the Zemplínska šírava reservoir (Fig. 2). Total PCB concentrations were generally associated with the trophic position of the species within a food chain. In the predatory species, pike and pike-perch, the concentrations in both years were particularly high, which was expected for the highest trophic level (Table 1). The bottom-feeding and detritivorous non-predatory freshwater bream that obtains nutrients directly from the sediments exposed to PCBs, however, had comparable PCB concentrations. In contrast, the common carp that also feeds from the bottom sediments surprisingly had the lowest PCB concentrations, along with the European eel with a benthic feeding habit and a high body-fat content (Fig. 2). PCBs clearly tended to bioaccumulate in the muscle tissues of some species (freshwater bream, pike and pike-perch) during the five years (2004–2009) between analyses (Fig. 2). PCB concentrations increased significantly (t=-2.43, df=13, P<0.05) in these species and more than two-fold in freshwater bream. The opposite, but not significant, trend was seen in two cyprinids (common carp and goldfish), in which the PCB concentrations were lower in 2009 than in 2004 (Fig. 2).
PCB DISTRIBUTION ASSOCIATED WITH FISH TISSUES/ORGANS
The concentrations of the PCB congeners in the tissues/organs of both predatory and non-predatory fish were high (Table 2).The liver was the major target organ for PCBs in the predatory fish (Table 2), and it received the largest proportion (24%) of the overall contaminant burden. The PCB concentrations (lipid wt) in the tissues/organs decreased in the order: liver > muscles > adipose tissue > hard roe > kidney > bones > brain, with congener 153 markedly dominant in all tissues/organs of the predatory fish. The concentrations of all PCB congeners were significantly lower in the brain and in the liver and muscles, which had the highest concentrations, except for congener 28 in the muscles. Hard roe had the highest PCB accumulation capacity in the non-predatory fish, followed by adipose tissue, bones, liver, muscles, kidney and brain. Similarly to the predators, the non-predators had high mean concentrations of congener 153 in all tissues/organs. Despite the considerable variation in PCB concentrations in the various tissues/organs, the observed differences were not significant in the non-predatory fish (P>0.05; Table 2). The two-way main-effect ANOVA indicated that the feeding habits of the fish [F(6, 77)=9.10, P<0.00001] and particularly the individual fish tissues/organs [F(36, 340.89)=1.77, P<0.01] affected PCB bioaccumulation. PCB concentrations detected in the muscles and liver were nearly two-fold higher in the predators, which prefer a diet of live fish, compared to the non-predatory fish (Table 2). The concentrations markedly exceeded the acceptable PCB limits established for fish by the Food Codex [31] in the muscle tissues for both groups of fish. The tissue/organ analysis performed in 2009 showed that fish accumulated PCBs selectively. The liver (hepatopancreas in cyprinids) had the highest PCB burden, with maximum concentrations recorded in northern pike (214.0 mg kg-1 lipid wt) and freshwater bream (163.0 mg kg-1 lipid wt) (Table 3)
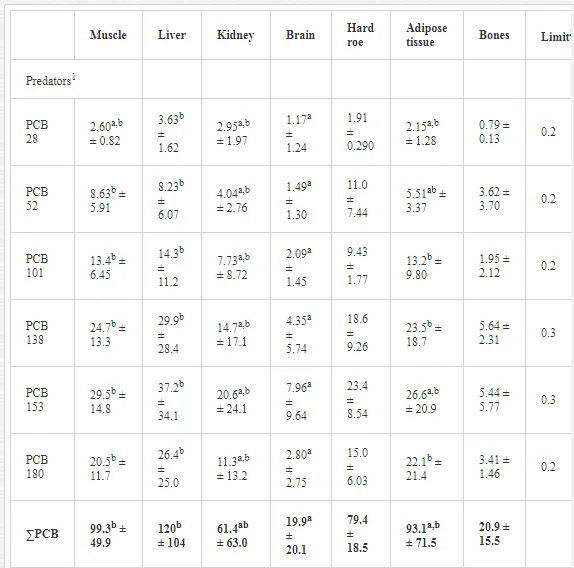


The storage capacity of the muscles was slightly lower, but in some cases, PCB concentrations were higher in the muscles than in the liver, as in the European perch (108.0 and 80.5 mg kg–1 lipid wt in muscles and liver, respectively) and goldfish (38.0 and 32.6 mg kg–1 lipid wt, respectively). PCBs accumulated to a lesser degree in the kidney and brain than in the muscle tissue and liver (Table 3), with a significant difference only for the brain (P<0.05). Most organic pollutants in aquatic ecosystems tend to accumulate in organisms, in which they can reach levels hundreds or thousands of times higher than the levels in water. Fish are at the top of the aquatic food web and concentrate high amounts of contaminants, including PCBs [6]. Ecotoxicological studies that have used fish as bioindicators have highlighted that the bioaccumulation of organic pollutants is affected substantially by biological factors, such as species, age, size, physiological condition and also parasitism, which was revealed recently [32, 33]. The position of fishes in a food chain and their lipid contents represent other important predictors of organochlorine accumulation [34, 35]
Fish are exposed to PCBs both via direct contact with the water and via food uptake by the membranes of the gastrointestinal tract [36]. Most of the PCBs, which have low solubility in water and fish, are likely derived from food rather than the ambient environment. Predatory fish, by eating other contaminated fish, accumulate higher amounts of organochlorine pollutants than fish with other feeding habits. Some authors [37], however, have arrived at opposite conclusions, and some studies have reported only slight differences between predators and bottom feeders [38, 39]. The results obtained in the present study are not entirely unequivocal; PCB concentrations were highest in the predatory perch, pike and pike-perch, but the non-predatory freshwater bream had comparable concentrations. The feeding behavior of bream is characterized by permanent direct contact with the sediments and a small radius of migration. Bream were thus once considered an important transfer vector for PCBs and one of the best organisms for monitoring freshwater contamination [40]. Our results support this finding.
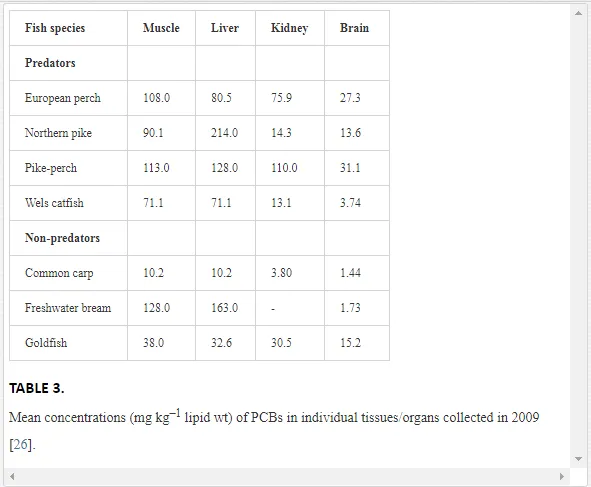
European eel is commonly classified as a predaceous carnivorous fish, although its position in the trophic scale is lower than that of typical predators such as pike and pike-perch [25]. PCBs are lipophilic compounds prone to high bioaccumulation in eels, which have a high fat content. Accordingly, PCB concentrations in eels are usually higher than in other freshwater fish species [34, 41]. In our study, the muscles of the European eel had an unexpectedly low level of total PCBs (17.1 mg kg–1 mean lipid wt), which is the second lowest level in the nine species examined. Due to the interrupted natural flow of groundwater, we assumed that the sedentary eels from the reservoir do not use their energy reserves to migrate upstream, and so may have stored PCBs for long periods in their metabolically inactive adipose tissues. Adipose tissue can retain PCBs for a long time, or PCBs may accumulate in other fat-rich tissues/organs, most probably the liver. We could not confirm the latter hypothesis, as we did not capture any eels in 2009, when the tissue-specific accumulation was investigated. The closely related cyprinids (carp, freshwater bream, goldfish and roach) with similar ecologies and food preferences [25] differed markedly from each other in PCB content. PCB concentrations were relatively similar in these fishes in 2004, ranging between 27.2 mg kg–1 lipid wt in carp and 62.5 mg kg–1 lipid wt in roach, but varied widely in 2009. Freshwater bream had extraordinarily high PCB concentrations in 2009 (128.0 mg kg–1 lipid wt). Species-specific differences in PCB concentrations were also found in the individual tissues/organs of the cyprinids. PCBs preferentially accumulated in the hepatopancreas in freshwater bream, but the concentrations were equal or nearly equal in the hepatopancreas and muscles of carp and goldfish, respectively (Table 3). These differences could not be attributed to different diets, because these species feed on similar foods (bottom-dwelling invertebrates, plankton in the deeper parts of water bodies, plants and detritus). In addition to biological indices, the environmental conditions prevailing at a particular field site must be considered, because even slight environmental perturbations can induce a high degree of heterogeneity in biochemical markers [42].
Among the potential causative factors responsible for the high variation of PCB levels in our fish, particularly the tissues/organs in the same species (compare pike, pike-perch, carp and bream in 2004 and 2009), the highly varying hydrological conditions in the reservoir may have played a large role. The regular monitoring of the reservoir from 1999 to 2007 [43, 44] revealed large variability in the deposition of sediments at different sites and even the movement of sediments (so-called bottom waves) during seasonal floods, which released PCBs from the sediments and became more available to aquatic organisms. The concentrations of PCB indicator congeners in the bottom sediments increased substantially between 1999 and 2003 (467 and 1633 µg kg–1 dry wt, respectively) but were much lower at the same sites in 2005 and 2007 (428 and 692 µg kg–1 dry wt, respectively) [43, 44]. The study by Šalgovičová and Zmetáková [20] is consistent with a role for hydrological conditions in PCB accumulation. PCB concentrations in their study showed a decreasing tendency in non-predatory fish species. The levels of PCBs in goldfish reached 133.7 mg kg–1 in 2002 but had decreased nine-fold by 2004. Most information about PCB compounds in aquatic organisms is from the muscles of fish and piscivorous birds [19, 45, 46], and very few data are available for the PCB contents in other organs. More attention has only recently been paid to the tissue-specific distribution of PCBs in fish [47]. The amount of total PCBs in a tissue or organ depends on the lipid content [19, 48]. The most important depot sites for PCBs in the predatory and non-predatory fish examined in the present study, next to the intestinal fat with obviously high lipid content (ca. 50%) [49], were the liver, hard roe and muscles. Other studies [50–52] have also reported higher PCB concentrations in the liver than in other organs, which could be due to its high fat content. Ovaries are also rich in fat and serve as important depots of contaminants such as PCBs [51]. The lipid and consequently PCB contents in hard roe, unlike in the liver, however, can vary considerably. During maturation of female fish, lipids pass from somatic to gonad tissue, accompanied by an increase in PCBs in the hard roe. In contrast, lipid-rich eggs released during spawning represent a major route of PCB loss in female fish [53]. The high PCB concentrations we found in the hard roe in both fish groups may thus be associated with the spawning period, because the majority of the fish in this study were caught in May, shortly before the peak of the spawn.
As shown by our results, the distribution of lipophilic compounds is not always directly proportional to the lipid content in individual tissues/organs. The brain, for example, with a relatively high fat content [54], appears to be much better protected against the accumulation of PCBs than other tissues/organs. This protection may be due to the efficient blood–brain barrier, as indicated by the relatively low concentrations of all investigated PCB congeners in our study, ranging from 0.84 (PCB 28 in non-predators) to 8.0 (PCB 153 in predators) mg kg–1 lipid wt. Several other conditions/factors, including species, age, size, feeding habits, metabolic activity of individual organs or the complex PCB transport in an organism may control PCB accumulation [26, 55].
CONCENTRATIONS AND PROFILES OF THE PCB CONGENERS IN FISH
The levels of six indicator congeners, 28, 52, 101, 180, 138 and 153 (in order of increasing concentrations), were high. All the congeners reached their upper acceptable limit in both predators and non-predators (Table 2). The various congeners were not distributed homogeneously within the tissues/organs. PCB 153 was present in higher concentrations than the other congeners in all tissues/organs, representing 31% and 34% of the mean total PCB concentrations in predators and non-predators, respectively (Table 2). PCB 138 and 180 accounted for approximately 25% and 21% of the total amount of PCBs in predators and non-predators, respectively.
Despite the differences in PCB concentrations in individual tissues/organs, the relative proportions of the congeners within the major target tissues/organs, the liver, hard roe, muscle, adipose tissue and kidney, were relatively similar in both fish groups. PCB 153 and 138, the congeners most frequently found in animal samples [56], were also predominant in our assays. Congeners 138, 153 and 180 are less hydrophobic and not as tightly bound to sediments as the more highly chlorinated octa-, nona- and deca-PCBs and are thus more readily available to aquatic organisms [57]. In addition, these congeners with chlorine atoms in positions 2, 4 and 5 in one (PCB 138) or both (PCB 153 and PCB 180) rings may be more resistant to metabolism and elimination from the organism than the congeners with fewer chlorine atoms, such as 28, 52 and 101 [58, 59]. The very high proportion of PCB 138, 153 and 180 found in our tissue/organ samples (76% and 79% of all PCB congeners in predators and non-predators, respectively) may reflect their low rates of biotransformation and resistance to metabolism.
PCB CONCENTRATIONS IN PERCH AND PARASITES
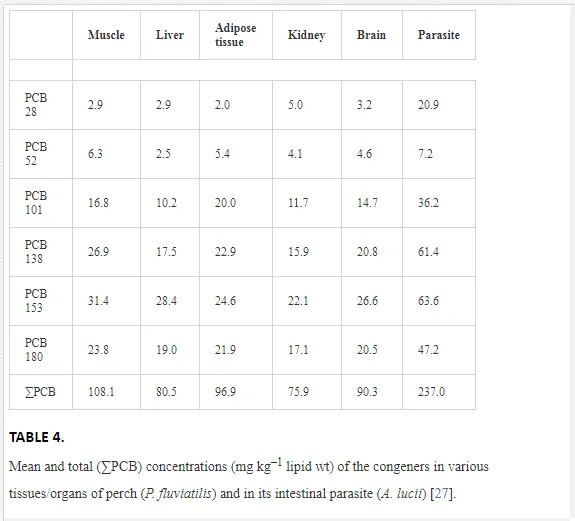
The accumulation of PCBs was studied in the perch–A. lucii host–parasite system. Acanthocephalans attached to the intestinal mucosa accumulated significantly higher concentrations of PCBs (single-means t-test, t<–4.2, P<0.001) than did the tissues/organs of their hosts (Fig. 3 and Table 4).

PCB 153 predominated in acanthocephalans (as in the fish), followed by PCB 138 and 180 (Table 4). Notably, the concentration of PCB 28 was approximately 10-fold higher in the parasites than in the muscles, liver and adipose tissue of the perch (Table 4).
The numbers of fish tissue/organ samples were too low for reliable statistical analysis (10 samples from infected and 14 samples from uninfected fish), but the results indicated much lower quantities of PCBs in all tissues/organs from the fish infected with acanthocephalans. The concentrations of PCBs were approximately 20- and 3-fold lower in the liver and muscles, respectively, of the infected perch (Fig. 3). Any kind of pollution represents environmental stress that influences host–parasite interactions and could cause outbreaks of fish diseases [60]. The immune system of fish is severely impaired by exposure to contaminants that consequently leads to an increase of parasitism. Sagerup et al. [61] found a positive correlation between PCB contamination of the habitat and intensity of nematode infection in gulls (Larus hyperboreus). Immunosuppression induced by PCBs was confirmed experimentally [62]. European eels infected with the swim-bladder nematode Anguillicola crassus were exposed to sublethal concentrations of PCB 126, which completely suppressed the antibody response in the eels. The fish exposed to the PCBs were consequently more easily infected than unexposed fish. A combination of anthropogenic stressors (e.g. PCB pollution) and natural stressors (e.g. parasitic infection) usually has a more detrimental effect on fish than each stressor alone [63]. Parasites are able to accumulate pollutants and consequently reduce their incorporation in the hosts [64, 65], particularly heavy metals. Supporting data for this role of parasites in fish PCB contamination, however, remain scarce. Pisidium amnicum, a freshwater clam, infected with larval stages of digeneans and exposed to organic pollutants (2,4,5-trichlorphenol (TCP) and benzo(a)pyrene) contained approximately 12% and 40% less TCP and benzo(a)pyrene, respectively, than uninfected clams [66]. Persson et al. [67], however, reported a slightly lower concentration of PCBs (1.92 ng g–1 lipid wt) in the tapeworm Eubothrium crassum than in their salmon (Salmo salar) hosts (2.65 ng g–1 lipid wt), which was attributed to the absence of an alimentary canal in all developmental stages of the tapeworms. The present study provides the first evidence of the ability of fish parasites (acanthocephalans) to accumulate relatively high levels of PCBs. Our data demonstrated lower PCB concentrations in all tissues/organs of infected relative to uninfected perch under natural conditions. These data indicate that some parasitic organisms may have a positive influence on their hosts in PCB-contaminated environments, as previously demonstrated for heavy-metal pollution [14, 65].
Conclusions
Our results demonstrated a persistent problem with the “old environmental heritage” of PCBs, leading to high risks mainly for predatory fish species in aquatic ecosystems, and for the human populations living near these regions. The commercial production of PCBs has been banned or severely restricted in Slovakia since the 1980s, but the fish in the Zemplínska šírava reservoir continue to accumulate these chemicals from the sediments polluted in the previous decades. Critical PCB levels recorded in fishes and the tendency of bioaccumulation in some species in the reservoir are essential for predicting which food webs are at risk for higher rates of bioaccumulation that endanger the health of upper-trophic predators, including humans living in the area. This study indicated that the different fishes, their dietary intakes and the chemical properties of the pollutants were interrelated factors, all of which were important in the tissue/organ burdens. Fish parasites, due to their good properties of bioaccumulation and position in the food chain, should be carefully considered in any ecotoxicological research.